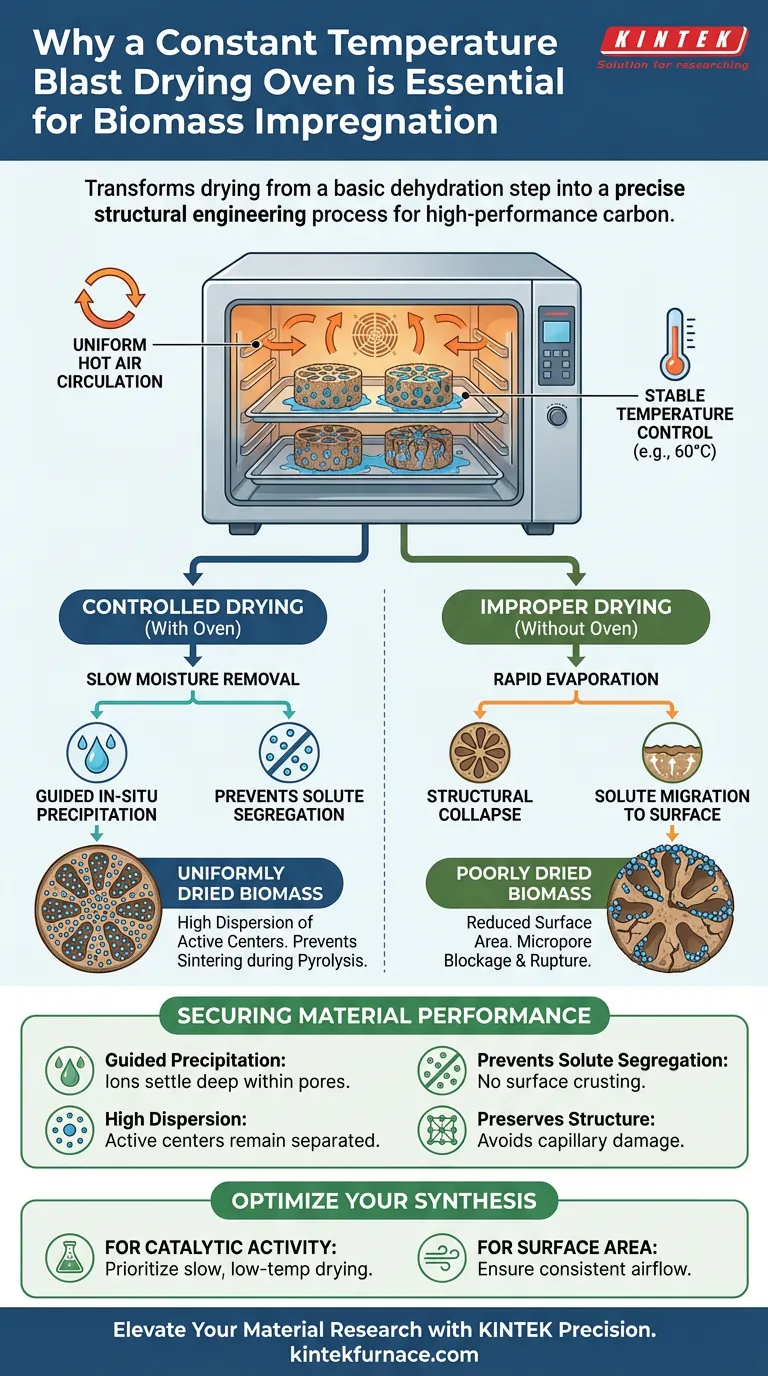
A constant temperature blast drying oven is an essential tool for biomass impregnation because it creates a uniform, circulating hot air environment that regulates the rate of moisture removal. This precise control allows for the slow, guided precipitation of metal precursor ions into the biomass pores, preventing them from clumping together or migrating to the surface during drying.
The oven transforms drying from a basic dehydration step into a precise structural engineering process. By controlling evaporation, it prevents solute segregation and structural collapse, ensuring that metal active centers remain highly dispersed for maximum performance during subsequent pyrolysis.

The Mechanics of Controlled Impregnation
Uniform Hot Air Circulation
The "blast" feature of the oven refers to forced air convection. This ensures that heat is distributed evenly throughout the chamber, eliminating cold spots.
For biomass materials soaked in precursor solutions, this uniformity is critical. It guarantees that the entire sample experiences the same thermal conditions, preventing uneven drying rates that could warp the material.
Regulating Moisture Removal
Simple heating can cause water to evaporate too quickly. A constant temperature oven allows you to set a stable, moderate heat (often around 60 °C) to facilitate slow moisture removal.
This controlled evaporation is necessary to manage the physical behavior of the liquid inside the biomass. It prevents the rapid phase changes that can damage delicate biological structures.
Securing Material Performance
Guiding In-Situ Precipitation
As the solvent evaporates, the metal ions dissolved in it must settle somewhere. The oven's controlled environment guides the in-situ precipitation of these ions.
By drying slowly, the ions are deposited uniformly within the porous structure of the biomass. This creates a homogeneous internal architecture rather than a chaotic coating.
Preventing Solute Segregation
Without controlled drying, dissolved materials tend to migrate to the surface as water evaporates, a phenomenon known as solute segregation.
The blast drying oven effectively prevents solute segregation. It ensures the metal precursors stay locked deep within the pores, rather than forming a crust on the exterior of the biomass.
Ensuring High Dispersion of Active Centers
The ultimate goal of impregnation is to prepare the biomass for pyrolysis (carbonization). The oven ensures the high dispersion of iron active centers.
If the drying is uniform, the metal ions remain separated. This separation prevents them from sintering (fusing) into large, ineffective clumps during the high-heat treatment of pyrolysis.
Understanding the Risks of Improper Drying
Structural Collapse
Biomass has a delicate microporous structure. If moisture is removed aggressively or unevenly, the capillary forces can cause the material structure to collapse.
This collapse reduces the surface area available for chemical reactions. The constant temperature environment mitigates this, preserving the structural integrity required for high-performance carbon materials.
Micropore Blockage
If the biomass is not dried thoroughly and uniformly before carbonization, residual pockets of water can vaporize instantly at high temperatures.
This rapid evaporation can lead to the blockage of micropores. It may also physically rupture the material, destroying the intricate pore network you are trying to create.
Optimizing Your Synthesis Protocol
To achieve the best results with your biomass carbon sources, tailor your drying approach to your specific performance goals.
- If your primary focus is Catalytic Activity: Prioritize slow, low-temperature drying to maximize the dispersion of metal active centers and prevent ion clustering.
- If your primary focus is Surface Area: Ensure consistent airflow to prevent pore collapse and avoid micropore blockage during the transition to carbonization.
By treating the drying phase as a critical control point rather than a passive step, you ensure the structural and chemical integrity of your final carbon material.
Summary Table:
| Feature | Impact on Biomass Impregnation | Benefit for Carbon Synthesis |
|---|---|---|
| Forced Convection | Eliminates cold spots and ensures uniform heat | Prevents warping and uneven drying rates |
| Constant Temp Control | Facilitates slow, managed evaporation | Avoids structural collapse and capillary damage |
| Guided Precipitation | Keeps metal ions deep within pores | Prevents solute segregation and surface crusting |
| Uniform Dispersion | Maintains separation of metal precursors | Prevents sintering and clumping during pyrolysis |
| Moisture Removal | Thorough dehydration before carbonization | Prevents micropore blockage and material rupture |
Elevate Your Material Research with KINTEK Precision
Don’t let improper drying compromise your carbon material's performance. KINTEK provides industry-leading laboratory solutions, including specialized Blast Drying Ovens, Muffle Furnaces, and Vacuum Systems, all engineered to preserve delicate microporous structures and ensure high-dispersion active centers.
Backed by expert R&D and manufacturing, our equipment is fully customizable to meet the unique needs of your biomass synthesis and carbonization protocols. Achieve superior catalytic activity and surface area today—Contact our specialists now to find the perfect thermal solution for your lab.
Visual Guide
References
- Wenxin Guo, Lichao Tan. Iron Active Center Coordination Reconstruction in Iron Carbide Modified on Porous Carbon for Superior Overall Water Splitting. DOI: 10.1002/advs.202401455
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1200℃ Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- Vacuum Hot Press Furnace Machine Heated Vacuum Press Tube Furnace
People Also Ask
- How does the post-rolling annealing process improve the interfacial microstructure? Enhancing Titanium-Steel Bond Strength
- What are the functions of a programmed temperature rise experimental system? Master Coal Pre-Oxidation Research
- What environmental conditions are critical for SiOC ceramicization? Master Precise Oxidation & Thermal Control
- How does a PID intelligent segmented temperature control system impact diamond tools? Precision Sintering Explained
- What is the significance of FE-SEM for SSBSN ceramics? Master Morphological Analysis for Superior Electrical Performance
- What is the primary purpose of the 600°C annealing treatment? Optimize Silver-Coated Ceramic Performance
- What are the advantages of using a vacuum oven for drying porous carbon? Protect Microstructures & Prevent Oxidation
- How do heating and stirring support chemical synthesis? Optimize Reaction Kinetics and Thermodynamics



















