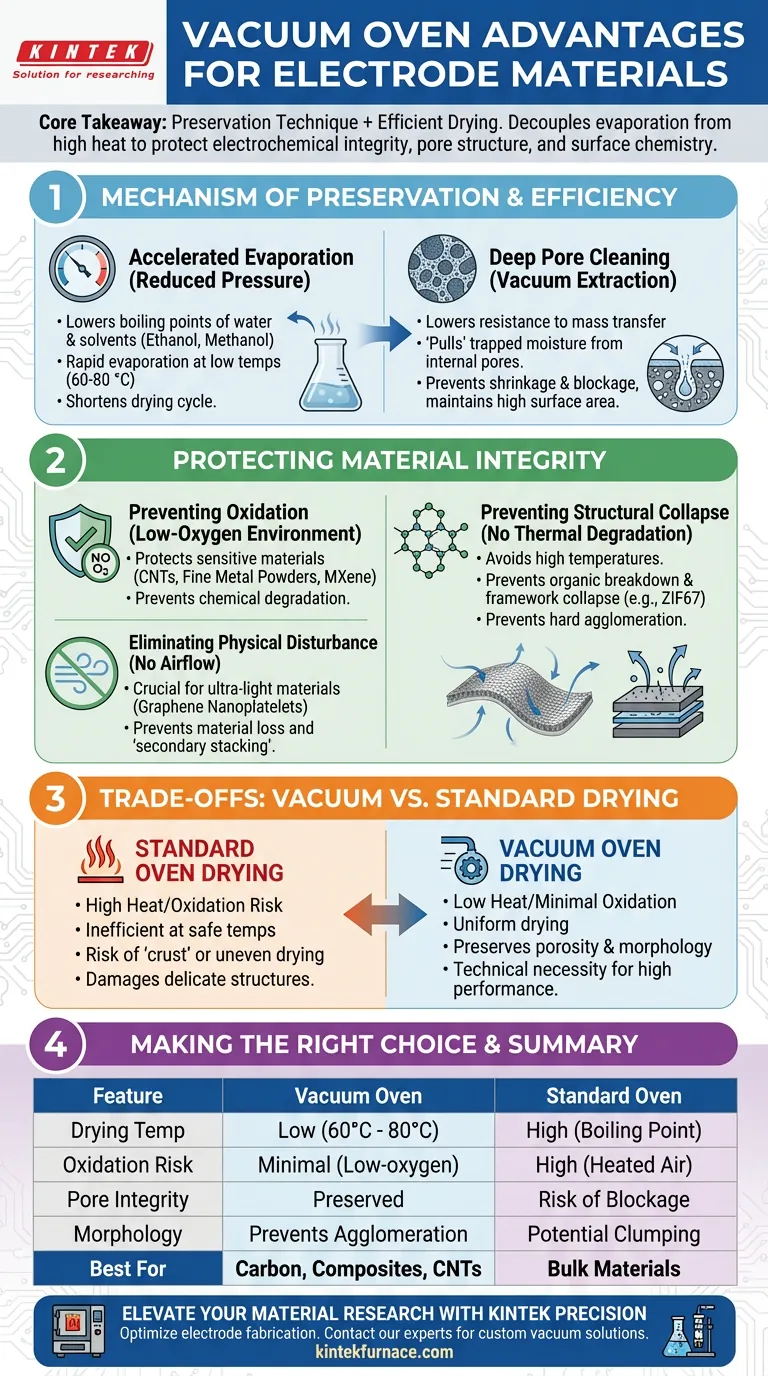
The primary advantage of using a vacuum oven for porous carbon and composite electrode materials is the ability to dry them rapidly at significantly lower temperatures (60 °C to 80 °C) by reducing environmental pressure. This process accelerates the evaporation of deep-seated moisture and solvents without subjecting the material to the thermal stress or oxidation risks associated with standard atmospheric ovens.
Core Takeaway Vacuum drying is fundamentally a preservation technique as much as a drying process. By decoupling evaporation from high heat, it protects the electrochemical integrity, pore structure, and surface chemistry of sensitive materials that would otherwise degrade in a standard oven.

The Mechanism of Preservation and Efficiency
Accelerating Evaporation via Reduced Pressure
In a standard oven, removing solvents often requires heating them to their boiling points at atmospheric pressure. A vacuum oven reduces the ambient pressure, which drastically lowers the boiling point of water and residual solvents like ethanol or methanol.
This allows these liquids to evaporate rapidly even at moderate temperatures (60 °C to 80 °C). This shortens the overall drying cycle significantly compared to atmospheric drying, which would require higher temperatures or longer times to achieve the same result.
Deep Pore Cleaning
Porous carbon and composite electrodes often contain moisture trapped deep within their internal structure. Standard thermal drying often struggles to evacuate these deep pores efficiently without excessive heat.
The vacuum environment lowers the resistance to mass transfer, effectively "pulling" water and solvents out of the internal pores. This prevents the shrinkage or blockage of active pores, ensuring the material maintains the high surface area required for effective ion transport in electrochemical applications.
Protecting Material Integrity
Preventing Oxidation
Standard ovens expose materials to heated air, which accelerates oxidation. This is particularly dangerous for materials like carbon nanotubes (CNTs), fine metal powders (e.g., Fe3Al), or MXene composites.
A vacuum oven operates in a low-oxygen environment. This prevents chemical reactions that degrade the material's performance, preserving the surface structure and chemical stability of nano-electrode materials.
Preventing Structural Collapse and Agglomeration
High temperatures in standard ovens can cause organic functional groups to break down or lead to the structural collapse of composite frameworks (like ZIF67).
Vacuum drying avoids this thermal degradation. Furthermore, it prevents the hard agglomeration of nanoparticles, ensuring that powders maintain their dispersibility and original microscopic morphology.
Eliminating Physical Disturbance
For ultra-light materials like Graphene Nanoplatelets, the airflow present in standard convection ovens can be destructive. It can blow the powder away or cause "secondary stacking," where layers clump together.
The vacuum environment eliminates airflow interference. This ensures that the physical arrangement of the filler remains intact and that no material is lost during the process.
Understanding the Trade-offs: The Risks of Standard Drying
While vacuum ovens require more complex equipment than standard laboratory ovens, understanding the specific risks of not using them is critical for electrode fabrication.
The Cost of Atmospheric Heat
Using a standard oven typically forces a choice between speed and quality. To dry quickly, you must raise the temperature, which risks oxidizing the active sites or collapsing the pore structure. If you lower the temperature to be safe, the drying process becomes inefficient, leaving residual solvents trapped in deep pores that can interfere with electrochemical performance.
The Integrity Gap
Standard drying often results in a "crust" or uneven drying where the surface dries before the core. In contrast, vacuum drying ensures uniformity. For high-quality molding and electrode performance, the porosity maintained by vacuum drying is not just a "nice-to-have"—it is often a technical necessity to prevent performance degradation.
Making the Right Choice for Your Goal
To select the right drying protocol for your specific material, consider the following technical priorities:
- If your primary focus is Chemical Stability: Utilize the vacuum oven to eliminate oxygen, which prevents the oxidation of sensitive components like CNTs and fine metal powders.
- If your primary focus is Electrochemical Performance: Rely on vacuum drying to prevent pore closure and shrinkage, ensuring maximum surface area for ion transport.
- If your primary focus is Morphology: Use the vacuum environment to prevent the hard agglomeration of nanoparticles and the physical displacement of ultra-light fillers like graphene.
Ultimately, the vacuum oven is the standard for electrode fabrication because it allows you to achieve complete solvent removal without compromising the delicate microstructures that drive performance.
Summary Table:
| Feature | Vacuum Oven Drying | Standard Oven Drying |
|---|---|---|
| Drying Temp | Low (60°C - 80°C) | High (Boiling point at 1atm) |
| Oxidation Risk | Minimal (Low-oxygen) | High (Heated air exposure) |
| Pore Integrity | Preserved (Vacuum extraction) | Risk of shrinkage/blockage |
| Morphology | Prevents agglomeration | Potential for clumping |
| Best For | Carbon, Composites, CNTs | Non-sensitive bulk materials |
Elevate Your Material Research with KINTEK Precision
Don't compromise the electrochemical integrity of your composite materials with outdated drying methods. Backed by expert R&D and manufacturing, KINTEK offers high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems, all customizable for your unique lab requirements.
Whether you are working with sensitive porous carbon or advanced graphene nanoplatelets, our vacuum solutions ensure uniform solvent removal without thermal degradation. Optimize your electrode fabrication today—Contact our experts to find your custom solution.
Visual Guide
References
- Serkan Demirel, Mehmet Hakkı Alma. High capacitive pt and NiOx loaded supercapacitors with commercial and green synthesized carbon-based materials. DOI: 10.1007/s10854-023-11885-7
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- Vacuum Heat Treat Sintering and Brazing Furnace
- Molybdenum Vacuum Heat Treat Furnace
- Vacuum Heat Treat Sintering Furnace Molybdenum Wire Vacuum Sintering Furnace
People Also Ask
- What role does a high-performance thermostatic oven play in determining the moisture content of sugar beet by-products?
- Why is a pre-melting process required in phase equilibrium studies? Reset Your Sample for Precise Results
- What is the function of magnetron sputtering equipment in Diamond/Cu composites? Enhance Bonding with Precision Coating
- What is the disadvantage of dental ceramic? Weighing Cost, Strength, and Aesthetics
- What is the function of aluminum foil in leather combustion experiments? Optimize Thermal Isolation and Accuracy
- Why is precise superheat temperature control required? Unlock High-Quality Soft Magnetic Nanocrystalline Alloys
- Why are deoxidizer powders sealed inside iron bolts? Achieve Precise Chemical Control in Steel Inclusion Preparation
- How do lab furnaces simulate fire environments for UHPFRC testing? Achieving ISO834 Standard Compliance



















