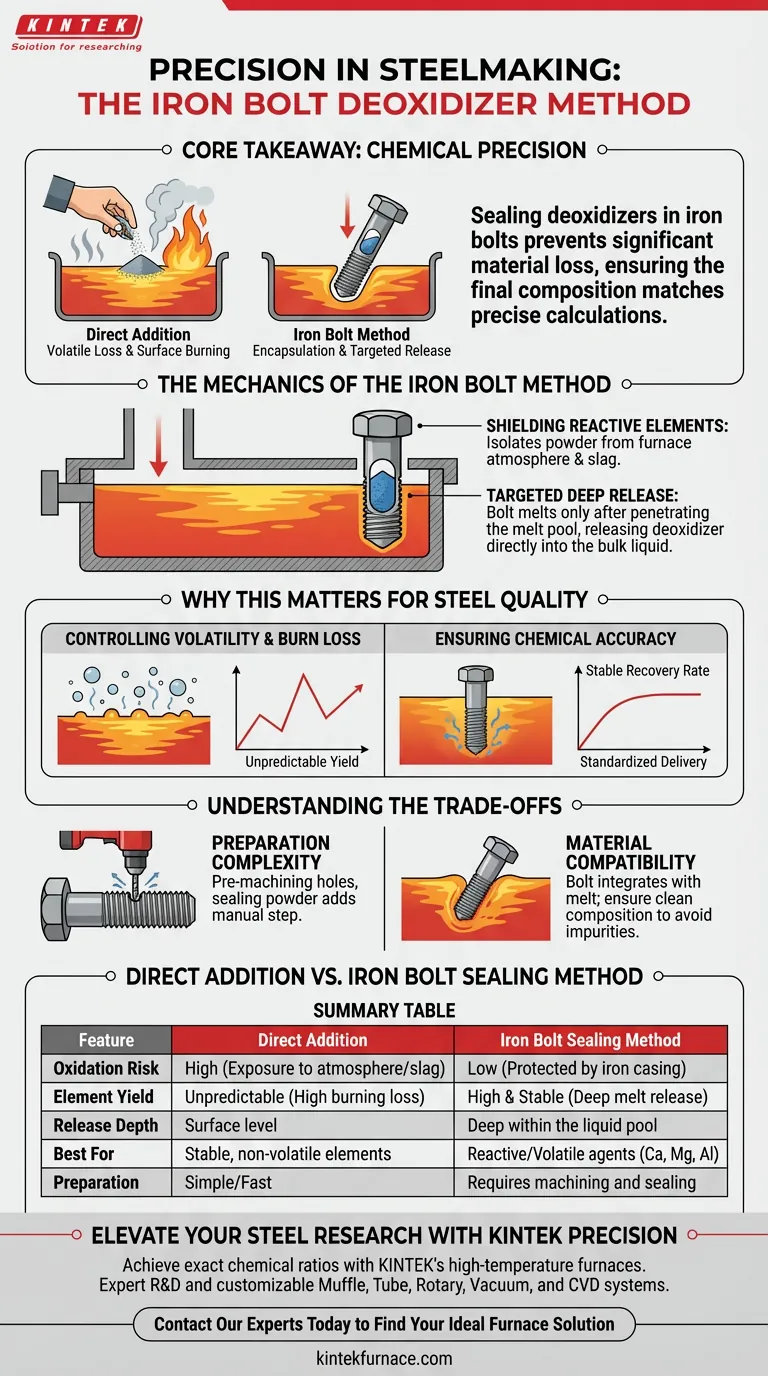
Sealing deoxidizer powders inside iron bolts is a critical technique for chemical precision. This method protects reactive or volatile additives, such as calcium-silicon alloy or aluminum powder, from oxidizing or evaporating before they enter the molten steel. By encasing these powders, the iron bolt acts as a delivery vehicle, melting only after reaching the depths of the liquid pool to release the agents exactly where they are needed.
Core Takeaway Direct addition of volatile powders often results in significant material loss due to surface burning and evaporation. Encapsulating these deoxidizers in iron bolts minimizes this loss, ensuring the final chemical composition matches your precise calculations.

The Mechanics of the Iron Bolt Method
Shielding Reactive Elements
Many deoxidizing agents are highly sensitive to oxygen. If exposed to the furnace atmosphere or the surface slag, they can ignite or react prematurely.
The iron bolt serves as a protective casing. It effectively isolates the powder from the harsh environment above the melt pool.
Targeted Deep Release
The physical weight and structure of the bolt allow it to penetrate the liquid iron surface. It does not melt instantaneously upon contact.
Instead, the bolt travels deep into the melt pool before the iron casing liquefies. This releases the deoxidizer directly into the bulk liquid, facilitating immediate and efficient mixing.
Why This Matters for Steel Quality
Controlling Volatility and Burn Loss
High temperatures in steelmaking cause certain elements to evaporate rapidly. This phenomenon, known as "burning loss," makes it difficult to predict how much additive will actually remain in the steel.
Sealing the powder prevents this surface-level evaporation. It ensures the additive is trapped within the liquid iron pressure, significantly improving the yield of the element.
Ensuring Chemical Accuracy
Inclusion preparation requires exact chemical ratios. Unpredictable losses turn this process into a guessing game.
By standardizing the delivery method, you stabilize the recovery rate of the deoxidizer. This leads to a final chemical composition that aligns strictly with your target specifications.
Understanding the Trade-offs
Preparation Complexity
This method is not as fast as bulk addition. It requires the pre-machining of holes into iron bolts.
You must also ensure the powder is sealed effectively within these cavities. This adds a manual preparation step to the workflow that must be accounted for in your timeline.
Material Compatibility
The delivery mechanism (the bolt) introduces mass into the melt. Since the bolt is iron, it generally integrates seamlessly with the steel melt.
However, one must ensure the bolt itself is clean and of a known composition to avoid introducing unintended impurities along with the deoxidizer.
Maximizing Precision in Inclusion Preparation
To ensure you are using this technique effectively, consider your specific experimental or production goals:
- If your primary focus is Compositional Accuracy: Use the iron bolt method for any high-volatility additives (like Ca or Mg) to eliminate the variable of evaporation loss.
- If your primary focus is Process Efficiency: Weigh the time cost of machining bolts against the cost of failed heats; for standard low-volatility additions, this method may be unnecessary over-engineering.
By sacrificing a small amount of time to prepare the bolts, you gain total control over the internal chemistry of your steel.
Summary Table:
| Feature | Direct Addition | Iron Bolt Sealing Method |
|---|---|---|
| Oxidation Risk | High (Exposure to atmosphere/slag) | Low (Protected by iron casing) |
| Element Yield | Unpredictable (High burning loss) | High & Stable (Deep melt release) |
| Release Depth | Surface level | Deep within the liquid pool |
| Best For | Stable, non-volatile elements | Reactive/Volatile agents (Ca, Mg, Al) |
| Preparation | Simple/Fast | Requires machining and sealing |
Elevate Your Steel Research with KINTEK Precision
Achieving exact chemical ratios in inclusion preparation demands more than just technique—it requires the right high-temperature environment. Backed by expert R&D and manufacturing, KINTEK offers a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems, as well as other lab high-temp furnaces, all customizable for your unique metallurgical needs.
Don't let burning loss compromise your results. Partner with KINTEK to ensure your lab processes are as precise as your calculations.
Contact Our Experts Today to Find Your Ideal Furnace Solution
Visual Guide
References
- Alejandra Slagter, Andreas Mortensen. Nanoindentation Hardness and Modulus of Al2O3–SiO2–CaO and MnO–SiO2–FeO Inclusions in Iron. DOI: 10.1007/s11661-024-07330-x
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- Stainless Steel Quick Release Vacuum Chain Three Section Clamp
- Silicon Carbide SiC Thermal Heating Elements for Electric Furnace
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- Chairside Dental Porcelain Zirconia Sintering Furnace with Transformer for Ceramic Restorations
People Also Ask
- How does plasma nitriding equipment improve the performance of titanium alloys in seawater? Boost Marine Durability
- What are the advantages of using a vacuum freeze dryer? Achieve Superior Morphology Control for Silica Nanoparticles
- Why is the mechanical mixing of precursor powders necessary for ITO thin films? Guide to Precision Growth
- How does a bias power supply influence AlCrSiWN coatings? Master Ion Bombardment for Superior Durability
- Why is preheating a metal mold to 660 °C necessary for Al/Cu bimetallic composites? Unlock Strong Chemical Bonding
- Importance of NaH2PO2 Layout in V-Ni3S2/NF Phosphorization: Ensuring Uniform 3D Doping
- How does a crucible furnace work? A Guide to Efficient Metal Melting
- What is the significance of applying full displacement constraints at fixed entry points? Ensure Thermal Accuracy




