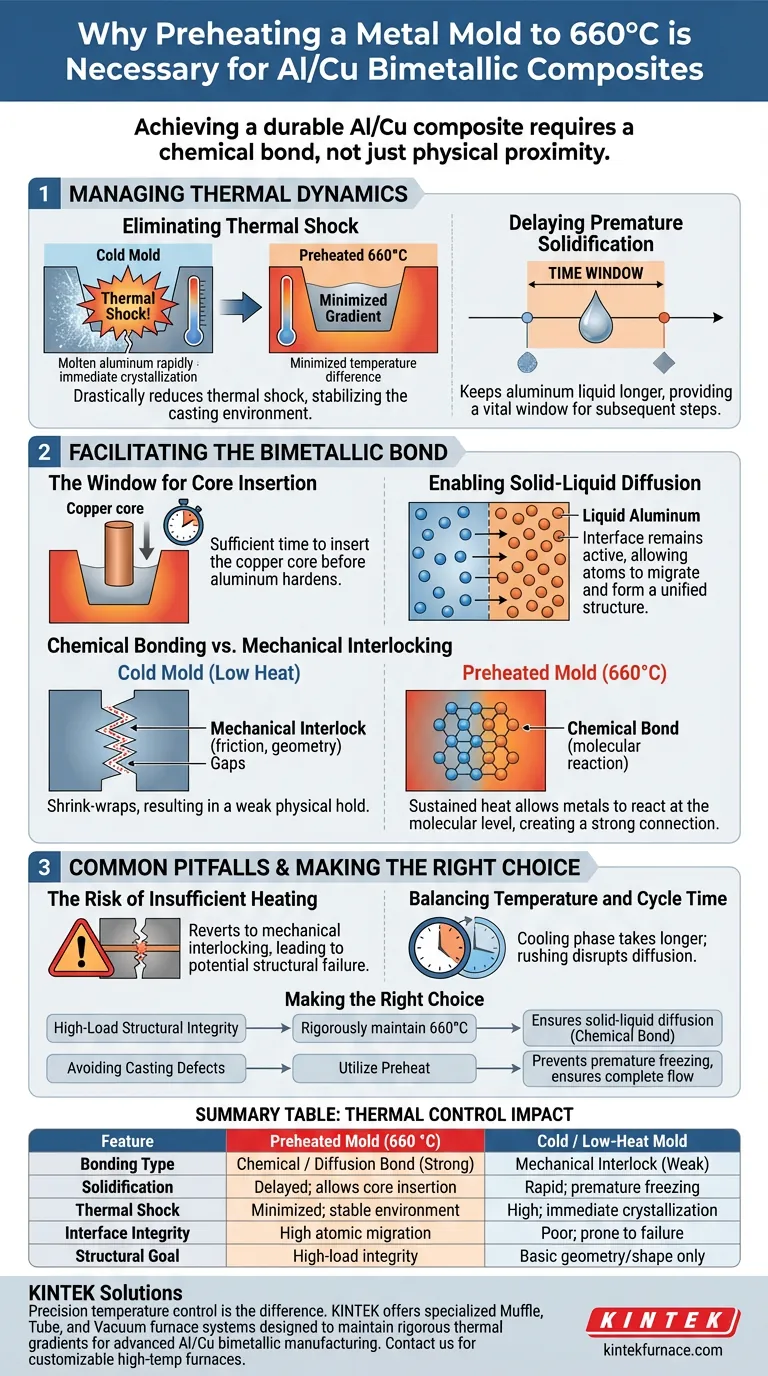
Preheating the metal mold to 660 °C is a critical process control measure designed to manage solidification timing and ensure metallurgical integrity. By maintaining the mold temperature near the melting point of aluminum, you prevent the molten metal from freezing immediately upon contact, creating the necessary thermal environment for a chemical reaction rather than a simple physical fit.
Achieving a durable Al/Cu composite requires more than physical proximity; it demands a chemical bond. Preheating the mold is the critical variable that delays solidification long enough to trigger a solid-liquid diffusion reaction at the interface.
Managing Thermal Dynamics
Eliminating Thermal Shock
When molten aluminum contacts a cold mold, the temperature difference causes rapid cooling and immediate crystallization at the mold walls.
Preheating the mold to 660 °C drastically reduces this thermal shock. It ensures the thermal gradient between the melt and the mold is minimized, stabilizing the casting environment.
Delaying Premature Solidification
The primary objective of this high preheat temperature is to keep the aluminum in a liquid state for a longer duration.
If the aluminum solidifies too quickly, the casting process freezes before the composite structure can be properly formed. This delay provides a vital time window for the subsequent processing steps.
Facilitating the Bimetallic Bond
The Window for Core Insertion
Manufacturing these composites often involves inserting a copper core into the aluminum melt.
Because the preheated mold delays solidification, there is sufficient time to insert the solid copper core before the aluminum hardens around it. Without this delay, the insertion would be physically impossible or would result in significant defects.
Enabling Solid-Liquid Diffusion
The most critical reason for preheating is to enable diffusion between the solid copper and the liquid aluminum.
At 660 °C, the interface between the two metals remains active, allowing atoms to migrate across the boundary. This atomic movement is necessary to form a unified structure.
Chemical Bonding vs. Mechanical Interlocking
Without preheating, the aluminum would shrink-wrap around the copper, resulting in a mechanical interlock. This is a weak physical hold that relies on friction and geometry.
Preheating facilitates a chemical bond. The sustained heat allows the metals to react at the molecular level, creating a significantly stronger and more reliable connection.
Common Pitfalls to Avoid
The Risk of Insufficient Heating
If the mold temperature falls significantly below 660 °C, the process reverts to mechanical interlocking.
You may achieve a casting that looks correct, but the interface will lack chemical continuity, leading to potential structural failure under stress or thermal cycling.
Balancing Temperature and Cycle Time
While high temperatures are necessary for bonding, they fundamentally change the production cycle.
Operators must account for the fact that the cooling phase will take longer. Attempting to rush the cooling after insertion can disrupt the diffusion process you worked to establish.
Making the Right Choice for Your Goal
To optimize your manufacturing process, align your temperature controls with your specific structural requirements:
- If your primary focus is High-Load Structural Integrity: rigorously maintain the mold at 660 °C to ensure the solid-liquid diffusion necessary for a true chemical bond.
- If your primary focus is Avoiding Casting Defects: utilize the preheat to prevent premature freezing, ensuring the aluminum flows completely around the copper core without gaps.
By treating temperature as an active ingredient in the bonding process, you transform a simple casting into a high-performance composite.
Summary Table:
| Feature | Preheated Mold (660 °C) | Cold / Low-Heat Mold |
|---|---|---|
| Bonding Type | Chemical / Diffusion Bond (Strong) | Mechanical Interlock (Weak) |
| Solidification | Delayed; allows core insertion | Rapid; premature freezing |
| Thermal Shock | Minimized; stable environment | High; immediate crystallization |
| Interface Integrity | High atomic migration | Poor; prone to structural failure |
| Structural Goal | High-load integrity | Basic geometry/shape only |
Precision temperature control is the difference between a weak mechanical fit and a high-performance chemical bond. Backed by expert R&D and manufacturing, KINTEK offers specialized Muffle, Tube, and Vacuum furnace systems designed to maintain the rigorous thermal gradients required for advanced Al/Cu bimetallic manufacturing. Whether you need a standard setup or a customizable high-temp furnace tailored to your unique casting needs, our technology ensures your materials achieve peak structural integrity. Contact KINTEK today to upgrade your lab's thermal processing capabilities!
Visual Guide
References
- Shima Ahmadzadeh Salout, S.M.H. Mirbagheri. Microstructural and mechanical characterization of Al/Cu interface in a bimetallic composite produced by compound casting. DOI: 10.1038/s41598-024-57849-7
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- Laboratory Quartz Tube Furnace RTP Heating Tubular Furnace
- Vertical Laboratory Quartz Tube Furnace Tubular Furnace
People Also Ask
- Why are different cooling methods compared for GFRP post-fire performance? Evaluate Thermal Shock & Safety Risks
- Why is it necessary to dry glassware in a 140 °C oven overnight before GTP? Ensure Precise Anhydrous Polymerization
- What advantages does tilting mirror technology provide for the growth of large-diameter crystals? Achieve Scale & Purity
- How does a high-precision Vertical Bridgman Furnace facilitate ZnGeP2 growth? Master Single Crystal Production
- How does a programmable high-temperature furnace improve the control of cooling rates? Enhance Ceramic Part Integrity
- What is the mechanism of bed powder in LLZO sintering? Optimize Lithium Stability and Phase Purity
- Why is Magnesium Hydride (MgH2) preferred for SiOx pre-magnesiation? Optimize Thermal Control and Battery Stability
- How does a high-pressure autoclave with a PTFE liner facilitate GLC synthesis? Ensure Purity and Safety



















