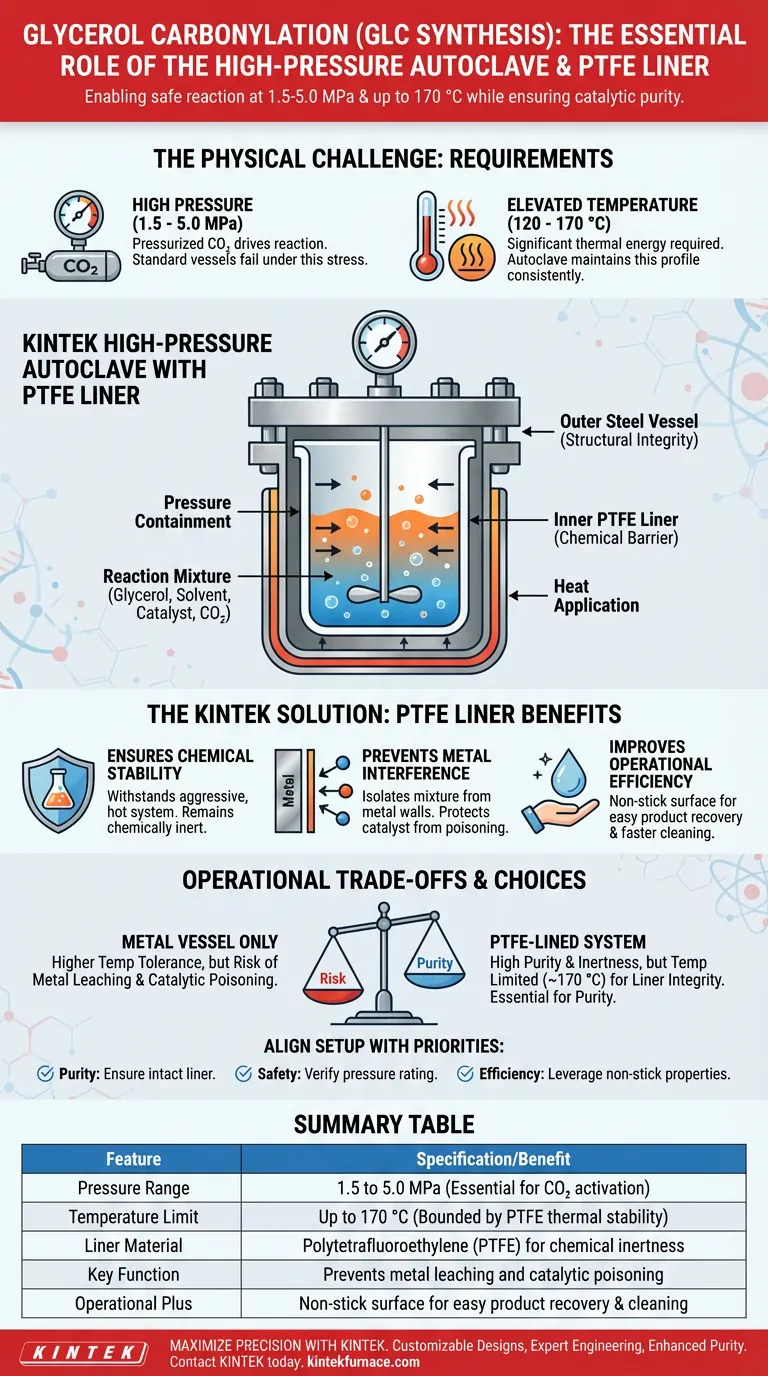
A high-pressure autoclave equipped with a polytetrafluoroethylene (PTFE) liner serves as the fundamental containment system for glycerol carbonylation (GLC synthesis). It allows the reaction to proceed safely under required pressures of 1.5 to 5.0 MPa while isolating the chemical mixture from the metal vessel walls. This setup is critical for maintaining catalytic purity and enduring temperatures up to 170 °C.
Core Takeaway GLC synthesis demands harsh physical conditions that standard laboratory glassware cannot withstand. The high-pressure autoclave provides the necessary structural integrity, while the PTFE liner acts as an essential barrier to prevent chemical contamination and catalytic interference from the metal reactor walls.

The Physical Requirements of the Reaction
Containing High Pressures
Glycerol carbonylation utilizes carbon dioxide as a reagent, which must be pressurized to drive the reaction.
The process specifically requires pressure conditions ranging from 1.5 to 5.0 MPa.
A standard reaction vessel would fail under this stress; the high-pressure autoclave is designed specifically to contain this force safely.
Managing Elevated Temperatures
In addition to high pressure, the synthesis requires significant thermal energy to proceed efficiently.
The operating window for this reaction lies between 120 °C and 170 °C.
The autoclave maintains this temperature profile consistently while keeping the pressurized gases contained.
The Critical Role of the PTFE Liner
Ensuring Chemical Stability
The reaction involves a hot glycerol and solvent system that can be chemically aggressive.
A PTFE liner provides a chemically stable surface that withstands this specific environment.
It remains inert even when exposed to the high temperatures required for the synthesis.
Preventing Metal Interference
Direct contact between the reactants and the metal walls of the autoclave can be detrimental.
The metal walls could chemically interact with the mixture, potentially interfering with the intended catalytic process.
The PTFE liner acts as a shield, isolating the reaction mixture to ensure that only the intended catalyst drives the synthesis.
Improving Operational Efficiency
Beyond chemical benefits, the liner offers practical advantages for the operator.
The non-stick nature of PTFE makes it significantly easier to collect the final products once the reaction is complete.
It also simplifies the cleaning process, allowing for faster turnover between experiments.
Operational Considerations and Trade-offs
Thermal Limitations vs. Chemical Inertness
While the PTFE liner provides essential chemical protection, it is the limiting factor regarding temperature compared to a bare metal vessel.
The reaction is capped at the operational limits of the polymer (around 170 °C in this context) to strictly maintain the liner's integrity.
Exceeding these temperatures could deform the liner, exposing the reactants to the metal walls and compromising the experiment.
The Necessity of the Liner
One might be tempted to forgo the liner to utilize the higher thermal tolerance of the steel autoclave.
However, doing so introduces the risk of metal leaching or catalytic poisoning.
The trade-off for purity is the strict adherence to the temperature limits imposed by the PTFE material.
Making the Right Choice for Your Goal
To ensure successful glycerol carbonylation, you must align your equipment setup with your specific experimental priorities.
- If your primary focus is Reaction Purity: Ensure the PTFE liner is intact and used in every run to prevent metal wall interference with the catalyst.
- If your primary focus is Process Safety: Verify the autoclave is rated for pressures exceeding 5.0 MPa to provide a safety margin above the required reaction conditions.
- If your primary focus is Workflow Efficiency: Leverage the non-stick properties of the liner to maximize product recovery and minimize solvent usage during cleanup.
By combining the structural strength of steel with the chemical inertness of PTFE, you create the ideal environment for high-purity GLC synthesis.
Summary Table:
| Feature | Specification/Benefit |
|---|---|
| Pressure Range | 1.5 to 5.0 MPa (Essential for CO2 activation) |
| Temperature Limit | Up to 170 °C (Bounded by PTFE thermal stability) |
| Liner Material | Polytetrafluoroethylene (PTFE) for chemical inertness |
| Key Function | Prevents metal leaching and catalytic poisoning |
| Operational Plus | Non-stick surface for easy product recovery and cleaning |
Maximize Your Synthesis Precision with KINTEK
High-purity glycerol carbonylation requires the perfect balance of thermal control and chemical resistance. KINTEK provides industry-leading laboratory solutions tailored for these demanding environments. Backed by expert R&D and manufacturing, we offer a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems, alongside customizable high-pressure autoclaves designed for your unique research needs.
Our value to you:
- Customizable Designs: Tailor vessel liners and pressure ratings to your specific chemistry.
- Expert Engineering: Ensure safety with hardware rated for extreme physical conditions.
- Enhanced Purity: Protect your catalytic processes with high-grade PTFE shielding.
Contact KINTEK today to optimize your lab's high-temperature and high-pressure workflows!
Visual Guide
References
- Simon Lukato, Grzegorz Litwinienko. Enhancing the Green Synthesis of Glycerol Carbonate: Carboxylation of Glycerol with CO2 Catalyzed by Metal Nanoparticles Encapsulated in Cerium Metal–Organic Frameworks. DOI: 10.3390/nano14080650
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Hot Press Furnace Machine Heated Vacuum Press Tube Furnace
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- Vacuum Hot Press Furnace Machine for Lamination and Heating
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- 600T Vacuum Induction Hot Press Vacuum Heat Treat and Sintering Furnace
People Also Ask
- Why is a sealed heating vessel used with a stepped heating process to infiltrate sulfur? Maximize Li-S Battery Performance
- Why is 600 °C critical for ZIF-8 carbonization? Achieve Optimal Surface Area and Functional Group Retention
- What is the role of a laboratory oven in mushroom dehydration? Master Pre-Treatment for Precise Biochemical Analysis
- How does the speed-controlled motor in a high-pressure autoclave influence the yield of glucose from starch?
- What is the role of mortar grinding combined with heat gun processing in catalyst synthesis? Achieving PtCln Dispersion
- What role does an industrial box-type resistance furnace play in phosphor conversion? Powering Material Synthesis
- What role does sodium silicate (Na2SiO3) play as a phase transition additive? Optimize Molten Salt Separation
- How is mechanochemical grinding used in lithium battery recovery? Unlock Efficient Solid-State Material Repair



















