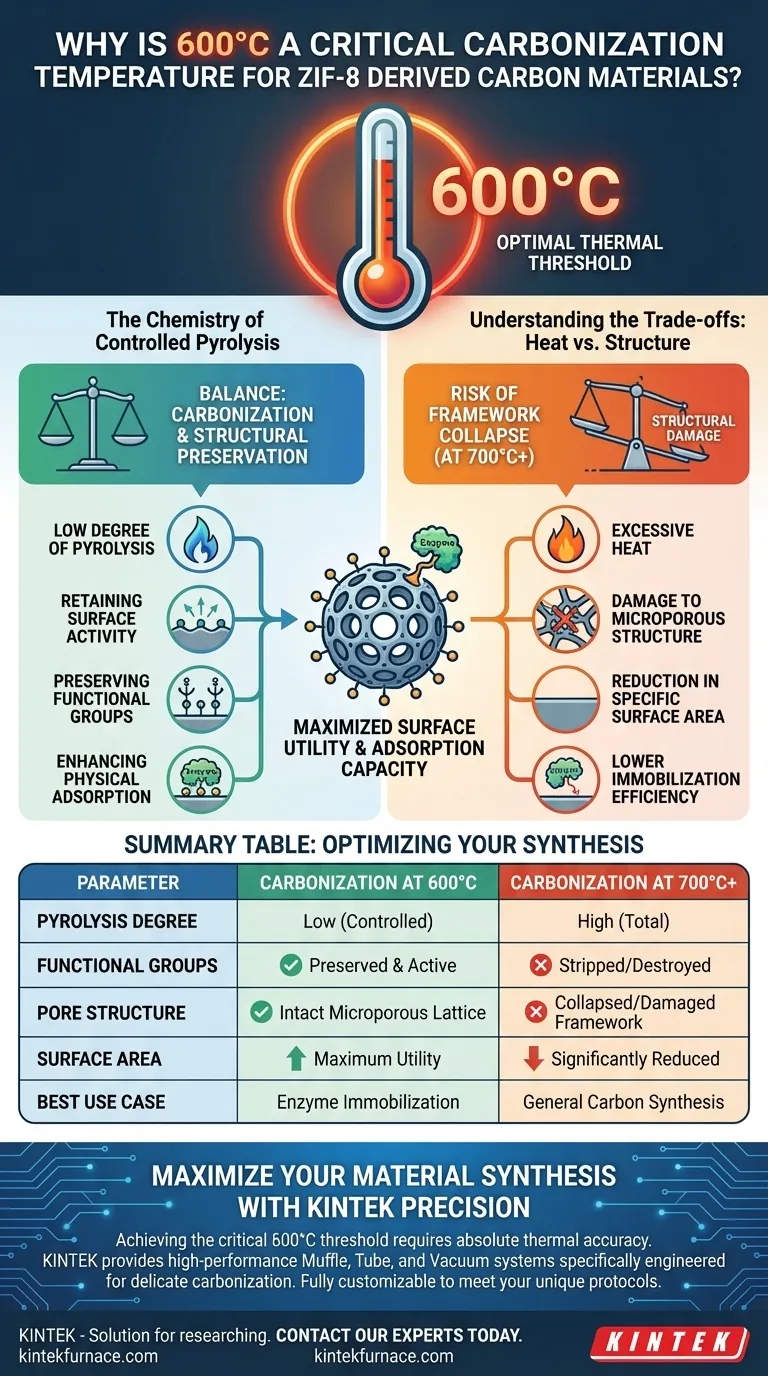
600 °C represents the optimal thermal threshold for synthesizing ZIF-8 derived carbon materials, particularly when the goal is maximizing surface utility and adsorption capacity. At this specific temperature, the material retains critical chemical properties that are frequently destroyed under more intense heating regimes, striking a balance between carbonization and structural preservation.
Carbonization at 600 °C maintains a low degree of pyrolysis, preserving vital surface functional groups and protecting the microporous framework. This balance is essential for applications requiring high physical adsorption, such as enzyme immobilization, which degrades significantly at higher temperatures.

The Chemistry of Controlled Pyrolysis
Retaining Surface Activity
The primary advantage of processing at 600 °C is the low degree of pyrolysis. Unlike higher temperatures which strip the material of its chemical identity, this temperature allows the ZIF-8 precursor to carbonize while retaining specific characteristics.
Preserving Functional Groups
Because the pyrolysis is not total, the process preserves surface functional groups. These chemical groups are not merely byproducts; they are active sites that facilitate interactions with other substances.
Enhancing Physical Adsorption
The retention of these functional groups creates a surface environment that is highly favorable for physical adsorption. For applications involving biological molecules, such as enzymes, these groups provide the necessary "anchors" to hold the molecules effectively.
Understanding the Trade-offs: Heat vs. Structure
The Risk of Framework Collapse
It is a common misconception that higher temperatures always yield better carbon materials. In the case of ZIF-8, exceeding 600 °C—specifically moving toward 700 °C or 800 °C—can compromise the material's integrity.
Damage to Microporous Structure
Excessive heat causes the delicate lattice of the ZIF-8 framework to break down. This thermal stress leads to damage of the microporous structure, effectively closing off the tiny pores that give the material its high utility.
Reduction in Specific Surface Area
As the structure collapses, there is a marked decrease in specific surface area. A lower surface area directly translates to less room for reactions or adsorption to occur.
Lower Immobilization Efficiency
The ultimate consequence of overheating is performance loss. Due to the reduced surface area and loss of functional groups, materials carbonized above 600 °C demonstrate lower immobilization efficiency.
Optimizing Your Synthesis Parameters
To ensure you are generating the most effective ZIF-8 derived carbon for your specific application, consider these guidelines:
- If your primary focus is Enzyme Immobilization: Adhere strictly to 600 °C to maximize the retention of surface functional groups and ensure high loading capacity.
- If your primary focus is Structural Integrity: Avoid temperatures of 700 °C or higher to prevent framework collapse and the loss of microporous volume.
Precision in temperature control is the deciding factor between a highly reactive substrate and a collapsed, inert carbon skeleton.
Summary Table:
| Parameter | Carbonization at 600 °C | Carbonization at 700°C+ |
|---|---|---|
| Pyrolysis Degree | Low (Controlled) | High (Total) |
| Functional Groups | Preserved & Active | Stripped/Destroyed |
| Pore Structure | Intact Microporous Lattice | Collapsed/Damaged Framework |
| Surface Area | Maximum Utility | Significantly Reduced |
| Best Use Case | Enzyme Immobilization | General Carbon Synthesis |
Maximize Your Material Synthesis with KINTEK Precision
Achieving the critical 600 °C threshold requires absolute thermal accuracy to prevent framework collapse. KINTEK provides high-performance Muffle, Tube, and Vacuum systems specifically engineered for the delicate carbonization of MOF-derived materials. Backed by expert R&D and manufacturing, our systems are fully customizable to meet your unique synthesis protocols, ensuring your ZIF-8 derived carbons maintain their vital functional groups and microporous integrity.
Ready to optimize your high-temp lab processes? Contact our technical experts today to find the perfect furnace for your research.
Visual Guide
References
- Yongheng Shi, Wei Du. Preparation of Ordered Macroporous ZIF-8-Derived Magnetic Carbon Materials and Its Application for Lipase Immobilization. DOI: 10.3390/catal14010055
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
People Also Ask
- Why is a silicone oil bath preferred for T5 aging of HPDC magnesium alloys? Precision Heat for Peak Strength
- How is a precision micro-Raman spectrometer utilized in the characterization of SSBSN ceramics? Master Phase Verification
- What is the purpose of using a precision oven for HAp-Fe3O4 drying? Achieve 110°C Stability & High Porosity
- Why is the mechanical mixing of precursor powders necessary for ITO thin films? Guide to Precision Growth
- Why is a constant temperature incubator required for 10-week fungal testing of Moso Bamboo? Ensure Testing Accuracy
- What are the benefits of using graphite or stainless steel crucibles for Rubidium Chloride? Ensure Maximum Purity
- How does a vacuum oven contribute to the performance of composite electrode slurries? Enhance Battery Life & Stability
- What are the limitations of functional group grafting through high-temperature heating? Achieve Chemical Precision



















