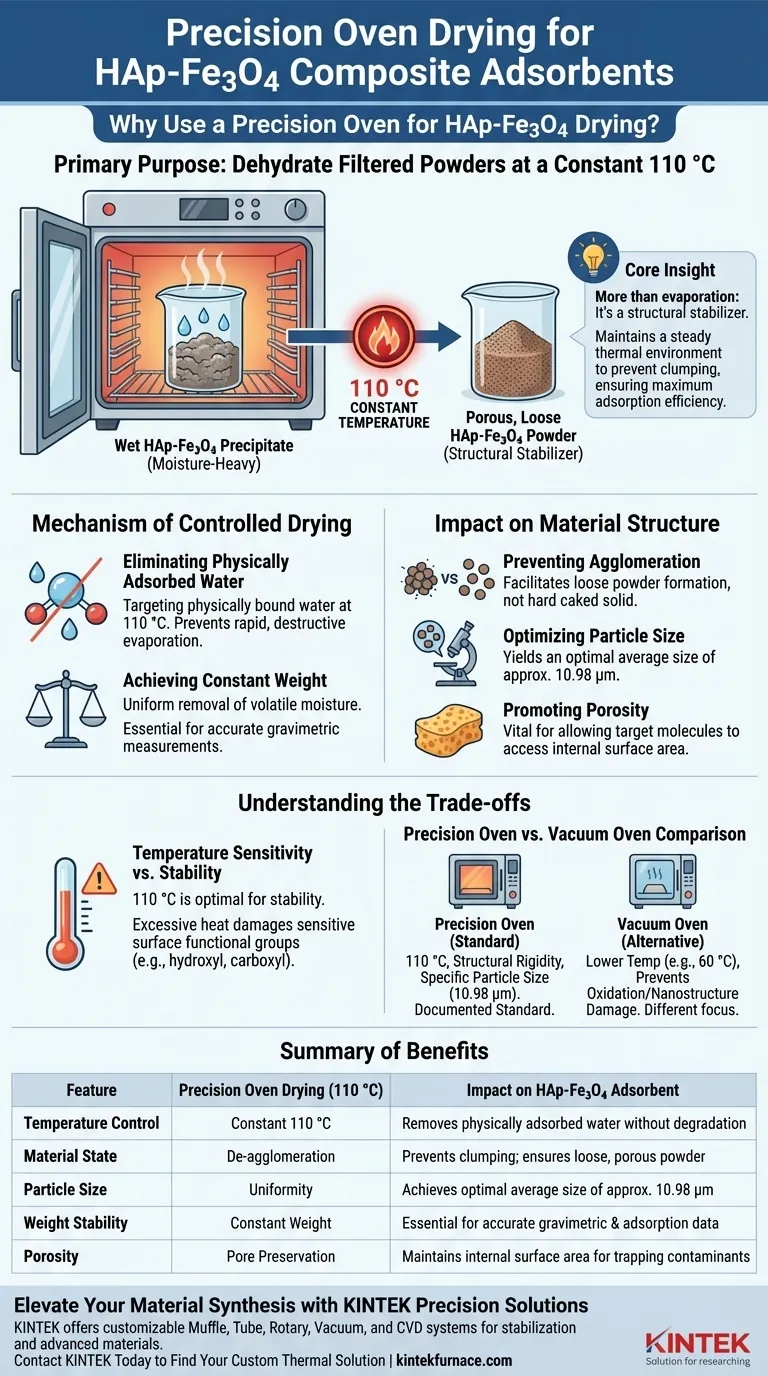
The primary purpose of using a precision oven in the drying stage of HAp-Fe3O4 composite adsorbents is to dehydrate filtered powders at a constant, controlled temperature of 110 °C. This specific thermal treatment removes physically adsorbed water without compromising the fundamental stability of the composite material.
Core Insight: The precision oven does more than simply evaporate water; it acts as a structural stabilizer. By maintaining a steady thermal environment, it prevents the powder from clumping, ensuring the final material remains loose and porous for maximum adsorption efficiency.

The Mechanism of Controlled Drying
Eliminating Physically Adsorbed Water
The synthesis of HAp-Fe3O4 involves wet filtration, resulting in a moisture-heavy filter cake.
The precision oven operates at 110 °C to target physically bound water molecules. This temperature is sufficient to drive off moisture but is controlled enough to prevent rapid, destructive evaporation.
Achieving Constant Weight
A critical aspect of using a precision oven is the ability to reach a constant weight.
By maintaining a stable temperature, the oven ensures that all volatile moisture is removed uniformly. This is essential for accurate gravimetric measurements in subsequent adsorption experiments.
Impact on Material Structure
Preventing Agglomeration
Drying is a critical phase where particles often stick together, reducing the surface area available for adsorption.
The precision oven process helps prevent this powder agglomeration. It facilitates the formation of a loose powder rather than a hard, caked solid.
Optimizing Particle Size
The controlled drying environment directly influences the physical dimensions of the adsorbent.
Proper use of the precision oven yields an average particle size of approximately 10.98 μm. This specific size indicates a successful transition to a usable powder form.
Promoting Porosity
Adsorbents rely on pores to trap contaminants.
By removing water gently yet thoroughly, the oven helps produce a porous adsorbent. This porosity is vital for allowing target molecules to access the internal surface area of the material.
Understanding the Trade-offs
Temperature Sensitivity vs. Stability
While 110 °C is optimal for structural stability in this context, thermal processing always carries risk.
You must ensure the temperature does not exceed the material's tolerance. Excessive heat can damage surface functional groups (such as hydroxyl and carboxyl groups), which are the active sites responsible for adsorption.
Precision Oven vs. Vacuum Oven
It is important to distinguish this method from vacuum drying.
A Vacuum Oven typically operates at lower temperatures (e.g., 60 °C) to prevent oxidation or nanostructure damage. However, for HAp-Fe3O4 composites requiring structural rigidity and specific particle sizing (10.98 μm), the standard precision oven at 110 °C is the documented standard for establishing the base material properties.
Making the Right Choice for Your Project
To maximize the effectiveness of your HAp-Fe3O4 adsorbent, align your drying method with your specific stability goals.
- If your primary focus is structural stability: Use the precision oven at 110 °C to ensure a loose, porous powder with an optimal particle size of ~10.98 μm.
- If your primary focus is surface chemistry protection: Monitor the duration of drying carefully to ensure you remove moisture without degrading sensitive hydroxyl or carboxyl active sites.
By strictly controlling the drying temperature, you transform a wet precipitate into a high-performance, porous adsorbent ready for application.
Summary Table:
| Feature | Precision Oven Drying (110°C) | Impact on HAp-Fe3O4 Adsorbent |
|---|---|---|
| Temperature Control | Constant 110 °C | Removes physically adsorbed water without degradation |
| Material State | De-agglomeration | Prevents clumping; ensures loose, porous powder |
| Particle Size | Uniformity | Achieves optimal average size of approx. 10.98 μm |
| Weight Stability | Constant Weight | Essential for accurate gravimetric & adsorption data |
| Porosity | Pore Preservation | Maintains internal surface area for trapping contaminants |
Elevate Your Material Synthesis with KINTEK Precision Solutions
Don't let inconsistent drying compromise your adsorbent's performance. Backed by expert R&D and manufacturing, KINTEK offers a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems, as well as specialized lab high-temperature furnaces. Whether you need to stabilize HAp-Fe3O4 composites or develop advanced porous materials, our equipment is fully customizable to meet your unique research needs.
Ready to optimize your lab's efficiency and material quality?
Contact KINTEK Today to Find Your Custom Thermal Solution
Visual Guide
References
- Charlena Charlena, Muhammad Dicky Iswara. Synthesis and Characterization of Hydroxyapatite Composites Based on Tutut (Belamya Javanica) and Magnetite by Coprecipitation as Adsorbents of Pb Metals Ion. DOI: 10.26554/sti.2025.10.1.111-122
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1200℃ Muffle Oven Furnace for Laboratory
- Vacuum Dental Porcelain Sintering Furnace for Dental Laboratories
- Vertical Laboratory Quartz Tube Furnace Tubular Furnace
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- Electric Rotary Kiln Pyrolysis Furnace Plant Machine Small Rotary Kiln Calciner
People Also Ask
- What is the purpose of using a vacuum drying oven? Maximize Drug Loading & Preserve Mesoporous Silica Nanoparticles
- Why is the enhancement of coke strength essential? Maximize Blast Furnace Efficiency & Stability
- What are the primary process objectives of using an infrared belt furnace? Optimize TOPCon Solar Cell Metallization
- What is the firing temperature for sintering? A Guide to Material-Specific Ranges
- What are the advantages of continuous furnaces? Boost Efficiency and Cut Costs in High-Volume Production
- What is the role of industrial electric drying ovens in FDSSC titanium photoanode treatment? Enhance Solar Efficiency
- What are the advantages of using an RTA system for CBTSe films? Precision Heating for Superior Thin Film Stoichiometry
- What is the necessity of an argon gas shielding system? Ensure Purity in Laser Remelting















