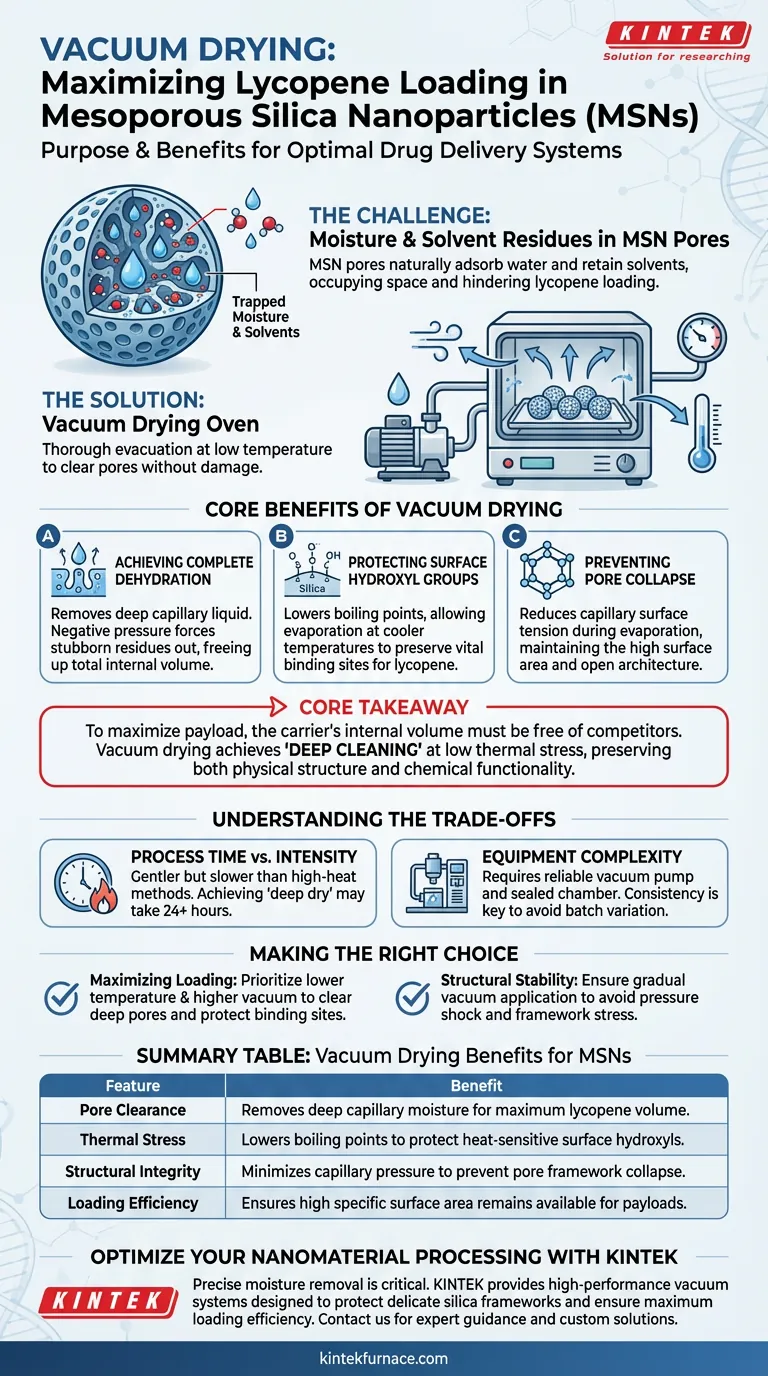
The primary purpose of using a vacuum drying oven is to thoroughly evacuate moisture and solvent residues from the internal pore structure of mesoporous silica nanoparticles (MSNs) without damaging the carrier.
By lowering the environmental pressure, this process allows liquids to evaporate at significantly reduced temperatures. This ensures the pores are completely empty and accessible for the subsequent impregnation of lycopene, maximizing the drug loading capacity.
Core Takeaway To maximize the loading of a payload like lycopene, the carrier's internal volume must be free of competitors like water molecules. Vacuum drying achieves this "deep cleaning" at low thermal stress, preserving both the physical pore structure and the chemical functional groups necessary for effective binding.

Preserving Integrity While Maximizing Volume
Achieving Complete Dehydration
The microscopic pores of mesoporous silica nanoparticles act as reservoirs that naturally adsorb water and retain solvents.
Standard drying methods often fail to remove liquid trapped deep within these capillary structures. A vacuum environment creates a negative pressure gradient that forces these stubborn residues to volatilize and exit the pores, ensuring the total internal volume is available for the incoming lycopene.
Protecting Surface Hydroxyl Groups
For many MSNs, the presence of surface hydroxyl groups is vital for interacting with and retaining the drug payload.
High-temperature drying can degrade or strip these functional groups. By utilizing vacuum pressure, the boiling point of trapped liquids is lowered, allowing for evaporation at cooler temperatures that leave these critical chemical "hooks" intact.
Preventing Pore Collapse
Drying a highly porous material creates significant capillary pressure, which can cause the delicate silica framework to shrink or collapse.
Vacuum drying mitigates this risk by reducing the surface tension forces during evaporation. This preserves the high specific surface area and the open pore architecture required to accommodate large molecules like lycopene.
Understanding the Trade-offs
Process Time vs. Intensity
While vacuum drying is gentler on the material, it is often a slower process compared to high-heat rapid drying. Achieving the necessary "deep dry" to clear micropores may require extended duration (often 24 hours or more) compared to standard convection ovens.
Equipment Complexity
Unlike standard ovens, this method requires a reliable vacuum pump and a sealed chamber capable of maintaining negative pressure. Leaks or pump fluctuations can lead to inconsistent drying, resulting in batch-to-batch variation in drug loading efficiency.
Making the Right Choice for Your Goal
To ensure optimal lycopene loading, align your drying parameters with your specific material requirements:
- If your primary focus is Maximizing Loading Capacity: Prioritize a lower temperature setting with a higher vacuum level to clear the deepest pores without thermally degrading surface binding sites.
- If your primary focus is Structural Stability: Ensure the vacuum is applied gradually to avoid sudden pressure changes that might stress the silica framework.
Vacuum drying is not just a cleaning step; it is a structural preservation technique that defines the upper limit of your drug loading efficiency.
Summary Table:
| Feature | Vacuum Drying Benefit for MSNs |
|---|---|
| Pore Clearance | Removes deep capillary moisture for maximum lycopene volume |
| Thermal Stress | Lowers boiling points to protect heat-sensitive surface hydroxyls |
| Structural Integrity | Minimizes capillary pressure to prevent pore framework collapse |
| Loading Efficiency | Ensures high specific surface area remains available for payloads |
Optimize Your Nanomaterial Processing with KINTEK
Precise moisture removal is critical for the success of your drug delivery systems. KINTEK provides high-performance vacuum systems designed to protect delicate silica frameworks and ensure maximum loading efficiency. Backed by expert R&D and manufacturing, we offer a comprehensive range of customizable Vacuum, Muffle, Tube, and CVD systems tailored for lab-scale research and industrial high-temp applications.
Ready to enhance your lab's efficiency and material integrity?
Contact KINTEK today for expert guidance and custom solutions!
Visual Guide
References
- Gabriela Corrêa Carvalho, Marlus Chorilli. Physicochemical characterization of a lycopene‐loaded mesoporous silica nanoparticle formulation. DOI: 10.1002/nano.202300131
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Heat Treat Sintering and Brazing Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- Vacuum Heat Treat Sintering Furnace Molybdenum Wire Vacuum Sintering Furnace
People Also Ask
- What role does the high-temperature boiling step play in rice husk silica conversion? Boost Your Extraction Yields
- Why is the preheating zone of a walking-beam furnace critical for Titanium/Steel clad plates? Minimize Thermal Stress
- Why is a pressurized environment necessary for HMF synthesis? Ensure Liquid Phase Stability at High Temperatures
- What role does an electric heating industrial furnace play in biomass pyrolysis? Unlock High-Quality Biochar Yields
- How does the use of carbon dioxide and a flow meter impact the physical activation of biochar? Master Pore Development
- Why is Magnesium Hydride (MgH2) preferred for SiOx pre-magnesiation? Optimize Thermal Control and Battery Stability
- What are the technical advantages of vacuum drying ovens for CeO2 separators? Protect Nanostructures & Boost Stability
- What is the primary role of the Thermal Oxidation (TO) process in Ti-6Al-4V ELI alloy? Enhancing Hardness and Wear



















