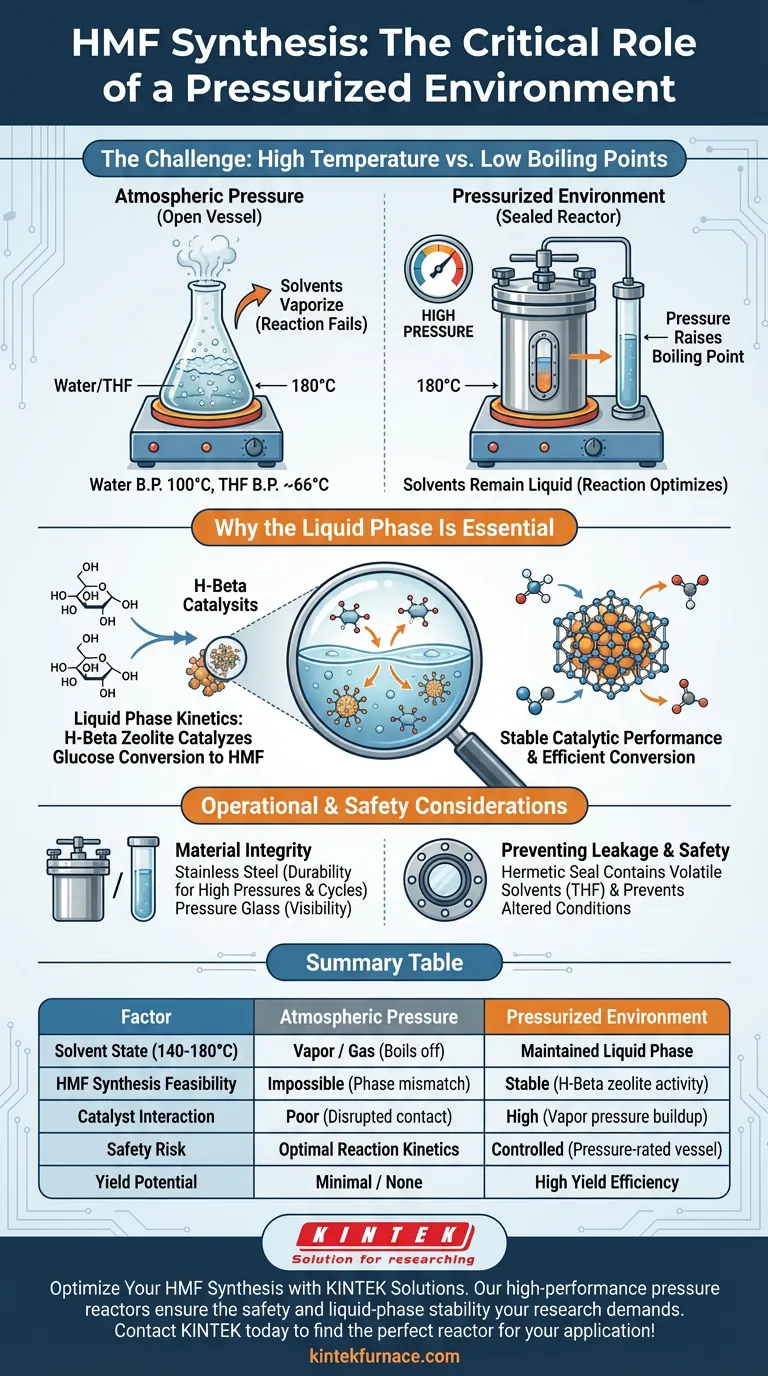
A pressurized environment is critical to maintain the solvent system in a liquid phase. Because HMF synthesis generally occurs at temperatures between 140°C and 180°C, the operating temperature significantly exceeds the standard boiling points of the solvents used, such as water and tetrahydrofuran (THF). Without a sealed, pressure-resistant vessel, these solvents would vaporize immediately, preventing the reaction from occurring.
By sealing the reactor, you artificially raise the boiling point of the solvent mixture. This forces the water and THF to remain in a liquid state despite the high heat, creating the necessary thermodynamic environment for the H-Beta zeolite to catalyze the conversion of glucose.

The Role of Temperature and Pressure
Overcoming Solvent Boiling Points
The synthesis of Hydroxymethylfurfural (HMF) relies on a biphasic solvent system, typically composed of water and THF.
Under standard atmospheric pressure, water boils at 100°C and THF boils at approximately 66°C.
Achieving High-Temperature Synthesis
To effectively convert glucose into HMF using H-Beta zeolite, the reaction requires temperatures ranging from 140°C to 180°C.
Since this range is far above the boiling points of the solvents, a pressurized vessel is the only way to conduct the reaction without losing the solvent to evaporation.
Thermodynamic and Catalytic Stability
Maintaining Liquid-Phase Kinetics
For the chemical conversion to proceed, the reactants (glucose) must interact with the catalyst (H-Beta zeolite) within a liquid medium.
Pressure-resistant vessels, such as stainless steel autoclaves or thick-walled glass tubes, confine the vapors. This containment generates internal pressure, keeping the solvent system in the liquid phase necessary for the reaction kinetics.
Ensuring Catalyst Performance
The stability of the catalytic process is directly tied to the consistency of the reaction environment.
The primary reference notes that a pressurized environment ensures stable catalytic performance. If the solvent were allowed to boil off or fluctuate between phases, the interaction between the zeolite and the glucose would be disrupted, leading to poor yields or catalyst deactivation.
Operational Considerations and Safety
Material Integrity
You must use stainless steel reactors or specialized pressure-resistant glass.
Standard laboratory glassware cannot withstand the internal pressure generated by heating solvents to 180°C. Using inadequate materials poses a significant risk of vessel rupture or explosion.
Preventing Solvent Leakage
Beyond maintaining pressure, the vessel must be hermetically sealed to prevent physical leakage.
Loss of solvent during the reaction alters the concentration of reactants and can render the thermodynamic conditions unstable. furthermore, containing THF (a volatile organic solvent) is essential for laboratory safety.
Applying This to Your Reaction Setup
To ensure successful HMF synthesis, select your equipment based on the following priorities:
- If your primary focus is reaction efficiency: Ensure your vessel is rated for pressures significantly higher than the vapor pressure of water/THF at 180°C to guarantee a stable liquid phase.
- If your primary focus is equipment longevity: Choose stainless steel over glass for repeated high-temperature cycles to minimize the risk of fatigue-related failure.
The pressure vessel is not just a container; it is an active component that enables the thermodynamics required for HMF synthesis.
Summary Table:
| Factor | Atmospheric Pressure | Pressurized Environment |
|---|---|---|
| Solvent State (140-180°C) | Vapor / Gas (Boils off) | Maintained Liquid Phase |
| HMF Synthesis Feasibility | Impossible (Phase mismatch) | Optimal Reaction Kinetics |
| Catalyst Interaction | Poor (Disrupted contact) | Stable (H-Beta zeolite activity) |
| Safety Risk | High (Vapor pressure buildup) | Controlled (Pressure-rated vessel) |
| Yield Potential | Minimal / None | High Yield Efficiency |
Optimize Your HMF Synthesis with KINTEK Solutions
Achieve the precise thermodynamic control required for Hydroxymethylfurfural (HMF) production with our high-performance pressure reactors. Backed by expert R&D and manufacturing, KINTEK offers a wide range of laboratory equipment including stainless steel reactors, pressure-resistant glass tubes, and high-temp furnace systems—all customizable for your unique lab needs.
Whether you are scaling up glucose conversion or researching H-Beta zeolite catalysis, our equipment ensures the safety, durability, and liquid-phase stability your research demands. Don't let solvent evaporation compromise your results.
Contact KINTEK today to find the perfect reactor for your application!
Visual Guide
References
- Xinyi Xing, Jianxiu Hao. H-Beta Zeolite as Catalyst for the Conversion of Carbohydrates into 5-Hydroxymethylfurfural: The Role of Calcination Temperature. DOI: 10.3390/catal14040248
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Multi Heating Zones CVD Tube Furnace Machine for Chemical Vapor Deposition Equipment
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- Split Chamber CVD Tube Furnace with Vacuum Station CVD Machine
People Also Ask
- What role does pack media play in the solid-state powder boriding process? Enhance Metal Hardness at High Temperatures
- Why is temperature control accuracy critical for K439B superalloy? Master 1160°C Solution Treatment
- What role does a laboratory oven play in the drying phase of Co–Mg catalyst precursors? Ensuring Component Uniformity
- What is the primary function of a laboratory blast drying oven? Essential Prep for La-EPS-C-450 Ceramic Adsorbents
- What are the core technical advantages of a flash sintering system? Elevate KNN Ceramic Manufacturing Performance
- What critical environmental conditions does a high-temperature recrystallization annealing furnace provide? Maximize Steel Strength
- What is the purpose of the long-term stabilization sintering step at 250°C? Secure Your CuO Nano-Network Integrity
- What role does the annealing process play in the post-treatment of stir-cast aluminum matrix composites? | KINTEK



















