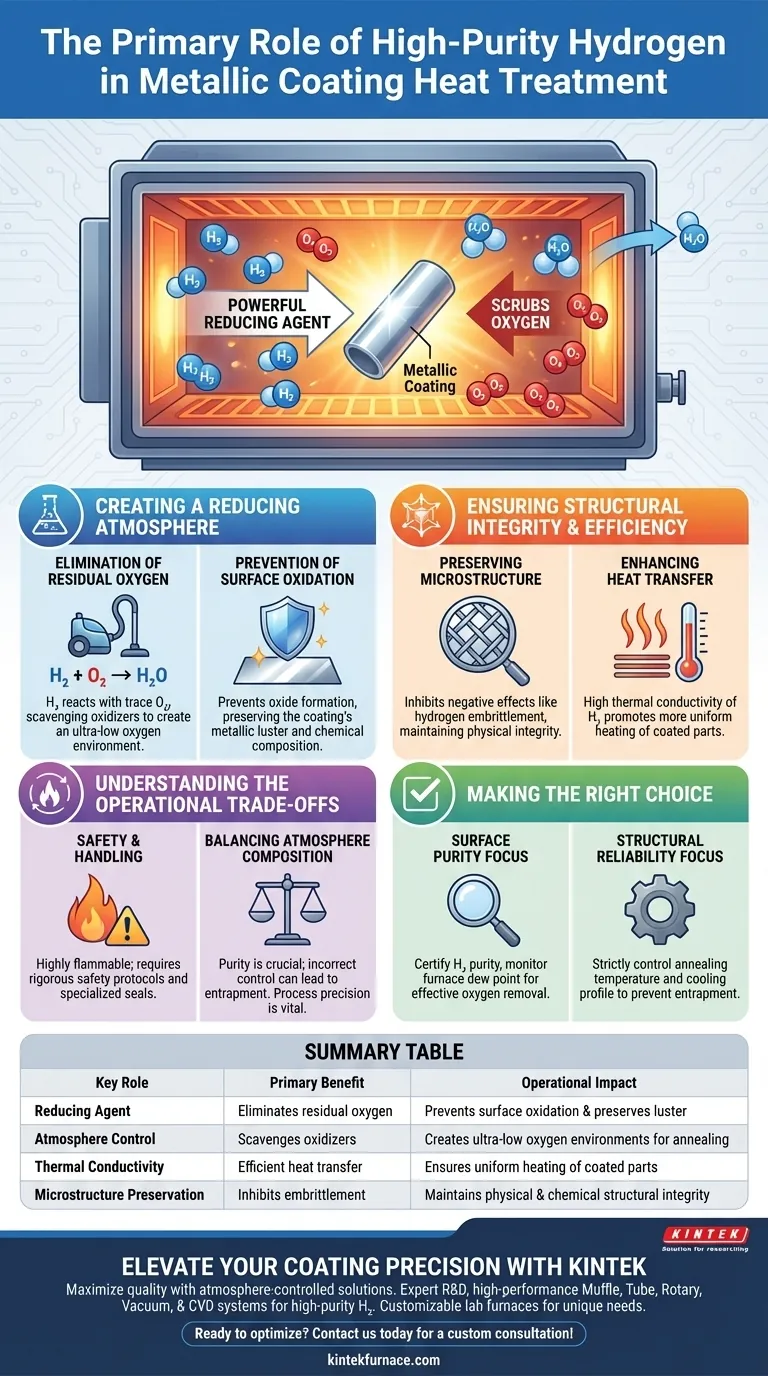
The primary role of high-purity hydrogen in heat treatment is to act as a powerful reducing agent. By actively reacting with and eliminating residual oxygen within the furnace, it creates an ultra-low oxygen environment essential for high-temperature annealing. This prevents the unintended oxidation of metallic coatings, ensuring their chemical purity and physical structural integrity are preserved throughout the process.
In metallic coating applications, hydrogen is not merely a passive atmospheric gas; it is an active chemical participant. Its ability to "scrub" oxygen from the environment is the defining factor that allows for the production of clean, defect-free metallic surfaces.

Creating a Reducing Atmosphere
Elimination of Residual Oxygen
The most critical function of hydrogen is its chemical reactivity with oxygen. Even in a sealed furnace, trace amounts of oxygen can remain.
Hydrogen reacts with this residual oxygen to form water vapor, effectively scavenging oxidizers from the environment. This reaction creates an oxygen-free or ultra-low oxygen atmosphere that is mandatory for high-quality metallic coatings.
Prevention of Surface Oxidation
Metallic coatings are highly susceptible to oxidation when exposed to high temperatures.
By maintaining a reducing atmosphere, hydrogen prevents oxides from forming on the coating's surface. This ensures that the coating retains its intended metallic luster and chemical composition rather than degrading into a dull, oxidized layer.
Ensuring Structural Integrity and Efficiency
Preserving Microstructure
Beyond surface aesthetics, the atmospheric composition affects the internal structure of the coating.
The primary reference notes that the presence of hydrogen helps inhibit negative effects, such as hydrogen embrittlement, in this specific context. By controlling the atmosphere, hydrogen helps maintain the physical integrity of the coating’s microstructure after the heat treatment is complete.
Enhancing Heat Transfer
While the primary goal is chemical reduction, hydrogen also offers thermal benefits.
As noted in supplementary contexts, hydrogen possesses high thermal conductivity. This property aids in heat transfer within the furnace, potentially allowing for more uniform heating of the coated parts.
Understanding the Operational Trade-offs
Safety and Handling
The use of high-purity hydrogen introduces significant safety considerations. Hydrogen is highly flammable and requires rigorous safety protocols and specialized furnace seals to prevent leaks and explosions.
Balancing Atmosphere Composition
While hydrogen is beneficial, the process relies on purity. If the hydrogen supply itself contains moisture or impurities, the reducing potential is compromised.
Furthermore, while the primary reference states hydrogen helps inhibit embrittlement in this context, incorrect cooling rates or saturation levels in other metallurgical contexts can lead to hydrogen entrapment. Process control is vital to ensure the gas improves integrity rather than compromising it.
Making the Right Choice for Your Goal
To optimize your heat treatment process for metallic coatings, consider the following regarding your atmosphere control:
- If your primary focus is Surface Purity: Ensure your hydrogen source is certified high-purity and monitor the furnace dew point to confirm that oxygen is being effectively converted and removed.
- If your primary focus is Structural Reliability: Strictly control the annealing temperature and cooling profile to allow the hydrogen to maintain the microstructure without becoming trapped within the metal lattice.
High-purity hydrogen is the industry standard for preventing oxidation, but its effectiveness relies entirely on the precision of your atmosphere control.
Summary Table:
| Key Role | Primary Benefit | Operational Impact |
|---|---|---|
| Reducing Agent | Eliminates residual oxygen | Prevents surface oxidation and preserves luster |
| Atmosphere Control | Scavenges oxidizers | Creates ultra-low oxygen environments for annealing |
| Thermal Conductivity | Efficient heat transfer | Ensures uniform heating of coated parts |
| Microstructure Preservation | Inhibits embrittlement | Maintains physical and chemical structural integrity |
Elevate Your Coating Precision with KINTEK
Maximize the quality of your heat treatment with atmosphere-controlled solutions from KINTEK. Backed by expert R&D and manufacturing, we offer high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems designed to handle high-purity hydrogen safely and effectively. Whether you require precise thermal conductivity or an ultra-low oxygen environment, our customizable lab high-temp furnaces are built for your unique industrial needs.
Ready to optimize your metallic coating process? Contact us today for a custom consultation!
Visual Guide
References
- Miqi Wang, Shi Fang. Enhancement in Corrosion and Wear Resistance of FeCoNiCrAl High-Entropy Alloy Coating Through Dual Heat Treatment with 3:1 N2/H2 Atmosphere. DOI: 10.3390/coatings15090986
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
People Also Ask
- How do quartz-capped vials facilitate the pyrolysis of magnetic chitosan carbon? Master Localized Reducing Atmospheres
- What are the functions of hot isostatic pressing (HIP) equipment? Achieve Peak Density in Powder Metallurgy
- What is the function of a high-temperature heat treatment furnace? Optimize AlCuCrFe2NiTi0.25 Alloy Properties
- Why use 10% Carbon Monoxide in black liquor pyrolysis? Prevent sodium volatilization for superior char quality.
- What role does a belt-type rapid sintering furnace play in forming metal contacts? Optimize Solar Cell Efficiency
- Why are SiC fragments added in microwave sintering? Boost Heating Uniformity and Prevent Cracks in Porous Ceramics
- What role does a high-temperature annealing furnace play in the preparation of AAO substrates? Enhance Pore Regularity
- What is the purpose of bottom-entry argon injection? Enhance Lithium-ion Battery Safety & Purge Efficiency

















