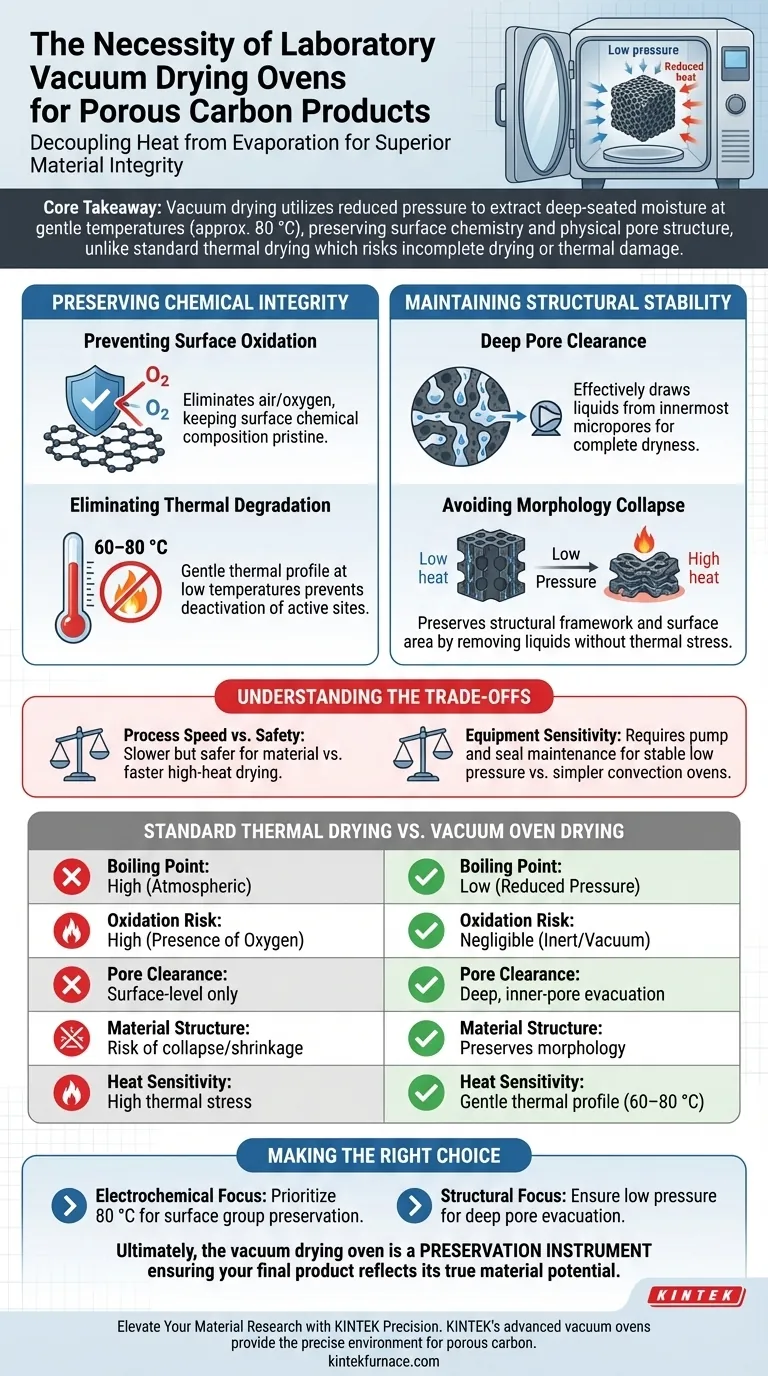
** The necessity of using a laboratory vacuum drying oven lies in its ability to decouple heat from evaporation.** By creating a low-pressure environment, the oven lowers the boiling point of water and residual solvents, allowing them to be removed efficiently at moderate temperatures (typically around 80 °C). This is critical for porous carbon materials, as it ensures deep moisture removal from within the pores without subjecting the material to high temperatures that would trigger oxidation or structural degradation.
Core Takeaway Standard thermal drying often forces a choice between incomplete drying or thermal damage. Vacuum drying solves this by utilizing reduced pressure to extract deep-seated moisture and solvents at gentle temperatures, preserving both the delicate surface chemistry and the physical pore structure required for high-performance applications.

Preserving Chemical Integrity
Preventing Surface Oxidation
When carbon materials are heated in the presence of air, they are susceptible to reacting with oxygen. This thermal oxidation can alter or destroy critical surface functional groups.
A vacuum drying oven eliminates this risk by removing the air (and thus the oxygen) from the chamber. This ensures the chemical composition of the carbon surface remains pristine during the drying process.
Eliminating Thermal Degradation
High temperatures are usually required to drive off moisture at standard atmospheric pressure. Unfortunately, this heat can degrade the intrinsic performance of carbon materials.
By lowering the pressure, the vacuum oven allows water and solvents to boil off at much lower temperatures, such as 60–80 °C. This gentle thermal profile prevents the deactivation of active sites on the material.
Maintaining Structural Stability
Deep Pore Clearance
Porous carbon relies on its open structure for performance, but moisture and washing solvents (like chloroform or acetone) often get trapped deep within these micropores.
The vacuum environment effectively draws these liquids out from the innermost pores. This ensures the material is truly dry, rather than just dry on the surface, which is vital for accurate electrochemical or adsorption testing.
Avoiding Morphology Collapse
Subjecting porous frameworks to high heat can cause the material’s morphology to shrink or collapse. This reduces the surface area and pore volume available for reactions.
Vacuum drying preserves the structural framework by removing liquids without the thermal stress that causes collapse. This maintains the physical "scaffold" necessary for optimal photocatalytic activity or catalyst adhesion.
Understanding the Trade-offs
Process Speed vs. Safety
While vacuum drying is safer for the material, it can be a slower process compared to high-heat flash drying. Users must balance the need for material integrity against the time required for a standard 12-hour cycle.
Equipment Sensitivity
Unlike standard convection ovens, vacuum ovens require the maintenance of seals and pumps to ensure a stable low-pressure environment. If the vacuum seal fails, moisture re-entry can occur immediately, compromising the sample.
Making the Right Choice for Your Goal
When finalizing your porous carbon processing, select your drying parameters based on your specific analytical needs:
- If your primary focus is Electrochemical Performance: Prioritize vacuum drying at 80 °C to prevent the oxidation of surface functional groups that drive reactivity.
- If your primary focus is Structural/Adsorption Analysis: Ensure the vacuum pressure is sufficiently low to evacuate all residual solvents from deep pores to prevent morphology collapse.
Ultimately, the vacuum drying oven is not just a drying tool; it is a preservation instrument that ensures your final product reflects the true potential of your material design.
Summary Table:
| Feature | Standard Thermal Drying | Vacuum Oven Drying |
|---|---|---|
| Boiling Point | High (Atmospheric Pressure) | Low (Reduced Pressure) |
| Oxidation Risk | High (Presence of Oxygen) | Negligible (Inert/Vacuum) |
| Pore Clearance | Surface-level only | Deep, inner-pore evacuation |
| Material Structure | Risk of collapse/shrinkage | Preserves morphology |
| Heat Sensitivity | High thermal stress | Gentle thermal profile (60–80 °C) |
Elevate Your Material Research with KINTEK Precision
Don't compromise your porous carbon's performance with subpar drying methods. KINTEK’s advanced laboratory vacuum ovens provide the precise environment needed to remove solvents while protecting delicate surface chemistries and pore structures.
Backed by expert R&D and manufacturing, KINTEK offers a full range of high-performance thermal solutions, including Muffle, Tube, Rotary, Vacuum, and CVD systems, all customizable for your unique lab requirements. Ensure the structural stability and chemical integrity of your materials today.
Contact our specialists now to find the perfect solution for your lab
Visual Guide
References
- Himanshu Gupta, Debasish Sarkar. Bitter Apple Pulp‐Derived Porous Carbon with Rich Oxygen Functionalities for High‐Performance Zinc‐Ion Storage. DOI: 10.1002/smll.202502071
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- Vacuum Heat Treat Sintering and Brazing Furnace
- 1200℃ Muffle Oven Furnace for Laboratory
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
People Also Ask
- What is the purpose of using nitrogen cylinders and flowmeters? Ensure Superior Carbon Fiber Recovery
- Why is stepped temperature control in a laboratory precision oven necessary? Mastering Porous TiCO Ceramic Curing
- How does a blast drying oven affect the preparation of BN-C precursors? Optimize Structural Stability and Homogeneity
- What is the importance of dynamic sealing in an InP crystal growth furnace? Ensure Pressure Integrity & Motion Control
- Why are rotary evaporators or industrial ovens recommended for handling Boron Carbide mixed slurries containing solvents?
- What factors should be considered when selecting a furnace based on material properties? Ensure Optimal Heat Treatment
- What are the energy consumption advantages of an industrial microwave pre-treatment system? Save Over 50% Energy
- What role does the vacuum system play in regulating the length of ZnO branches? Master Precision in Nanostructures



















