Stepped temperature control is essential to prevent the catastrophic collapse of the precursor structure. By utilizing four distinct temperature gradients ranging from 80°C to 180°C, a laboratory precision oven ensures the controlled evaporation of anhydrous ethanol while simultaneously allowing the phenolic resin to cross-link and solidify gradually.
Core Takeaway Rapid heating triggers violent solvent boiling that destroys the delicate matrix of ceramic precursors before they harden. A stepped thermal approach synchronizes solvent removal with polymer solidification, preserving a stable carbon skeleton necessary for high-quality porous TiCO ceramics.
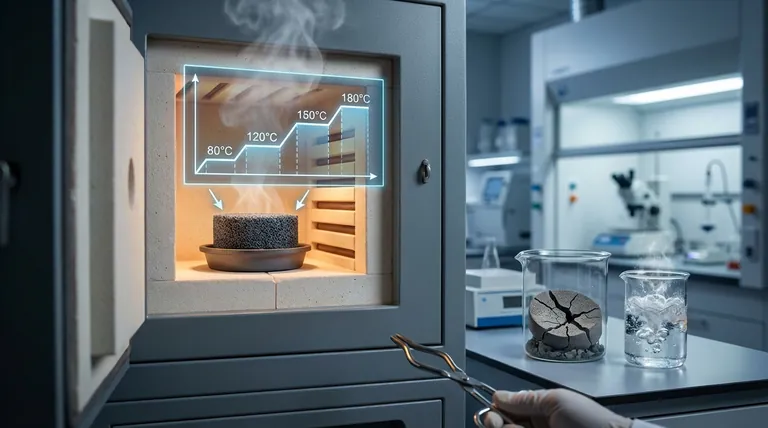
The Mechanics of Precursor Stabilization
Controlling Solvent Evaporation
The precursor mixture contains anhydrous ethanol, which acts as a solvent. If the temperature rises too quickly, this solvent will reach its boiling point abruptly.
Stepped temperature control modulates this process. It allows the ethanol to evaporate at a controlled rate, preventing the internal pressure buildup associated with flash boiling.
Synchronizing Resin Solidification
While the solvent evaporates, the phenolic resin within the mixture must undergo cross-linking. This is the chemical process that hardens the resin into a solid structure.
The temperature gradients (80°C to 180°C) are tuned to match the cure kinetics of the resin. This ensures the matrix creates a rigid framework capable of supporting itself as the solvent is removed.
Creating the Carbon Skeleton
The ultimate goal of this curing stage is to form a stable carbon skeleton. This skeleton serves as the foundation for the final ceramic material.
By carefully managing the heat, the process preserves the initial micron-scale pore structure. This porosity is the defining characteristic of the final TiCO ceramic product.
The Risks of Rapid Heating
Violent Solvent Boiling
Without stepped gradients, the ethanol transitions from liquid to gas explosively. The reference describes this as "violent solvent boiling."
This rapid expansion creates internal forces that the semi-liquid resin cannot withstand.
Structural Collapse
The primary failure mode in this process is the collapse of the precursor structure.
If the solvent leaves violently before the resin has sufficiently cross-linked, the voids collapse. This destroys the desired porosity and results in a dense, defective, or cracked material rather than a porous ceramic.
Optimizing the Curing Protocol
## Making the Right Choice for Your Process
To ensure the integrity of your porous TiCO ceramics, you must align your heating protocol with the physical limitations of your materials.
- If your primary focus is Structural Integrity: Adhere strictly to the four-step gradient starting at 80°C to prevent internal pressure from fracturing the matrix.
- If your primary focus is Pore Quality: Ensure the ramp rate allows for full solvent evacuation before the resin fully hardens to maintain open micron-scale pores.
Precision in the curing phase is the single most critical factor in defining the final architecture of the ceramic.
Summary Table:
| Curing Stage Factor | Requirement | Impact on TiCO Precursor |
|---|---|---|
| Temperature Range | 80°C to 180°C | Enables synchronized evaporation and solidification |
| Heating Method | 4-Step Gradient | Prevents violent solvent boiling and internal pressure |
| Solvent (Ethanol) | Controlled Removal | Maintains internal matrix without flash boiling |
| Phenolic Resin | Gradual Cross-linking | Creates a rigid, stable carbon skeleton structure |
| Pore Architecture | Micron-scale Retention | Preserves porosity for final high-quality ceramic |
Elevate Your Advanced Ceramic Processing with KINTEK
Achieving the perfect carbon skeleton requires uncompromising thermal precision. At KINTEK, we specialize in high-performance laboratory solutions tailored for complex materials science. Whether you are curing sensitive TiCO precursors or performing high-temp synthesis, our equipment delivers the exact temperature gradients your research demands.
Our value to you:
- Expert Engineering: Backed by world-class R&D and manufacturing.
- Versatile Solutions: A full range of Muffle, Tube, Rotary, Vacuum, and CVD systems.
- Tailored for Success: All systems are fully customizable to meet your unique laboratory protocols.
Don't let structural collapse compromise your results. Contact our specialists today to find the ideal precision oven or furnace for your application!
Visual Guide

References
- Xiaoyu Cao, Lei Feng. Microstructure, Mechanical Property and Thermal Conductivity of Porous TiCO Ceramic Fabricated by In Situ Carbothermal Reduction of Phenolic Resin and Titania. DOI: 10.3390/nano14060515
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1200℃ Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- Laboratory Muffle Oven Furnace with Bottom Lifting
People Also Ask
- How does a vacuum drying oven contribute to the study of the hydration degree in cement pastes? Essential Lab Insights
- What are the process advantages of using solution impregnation for PtS/Ti3C2Tx? Superior In-Situ Growth vs. Mixing
- What is the purpose of using a rotary evaporator or a vacuum drying oven? Preserving SiC Powder Quality Post-Milling
- What is the technical value of a Hydrogen Reduction-type Test Furnace in green steelmaking? Scale Sustainable Production
- How is induced heat generated in a conductive material exposed to a magnetic field? Master Rapid, Contactless Heating
- Why is precise temperature control programming indispensable for SFC research? Optimize Sintering Process Success
- What are the advantages of using a vacuum drying oven for MnMgPO4@C3N4? Preserving Photocatalyst Integrity
- What is the function of a laboratory oven in activated carbon preparation? Ensure Superior Material Stability



















