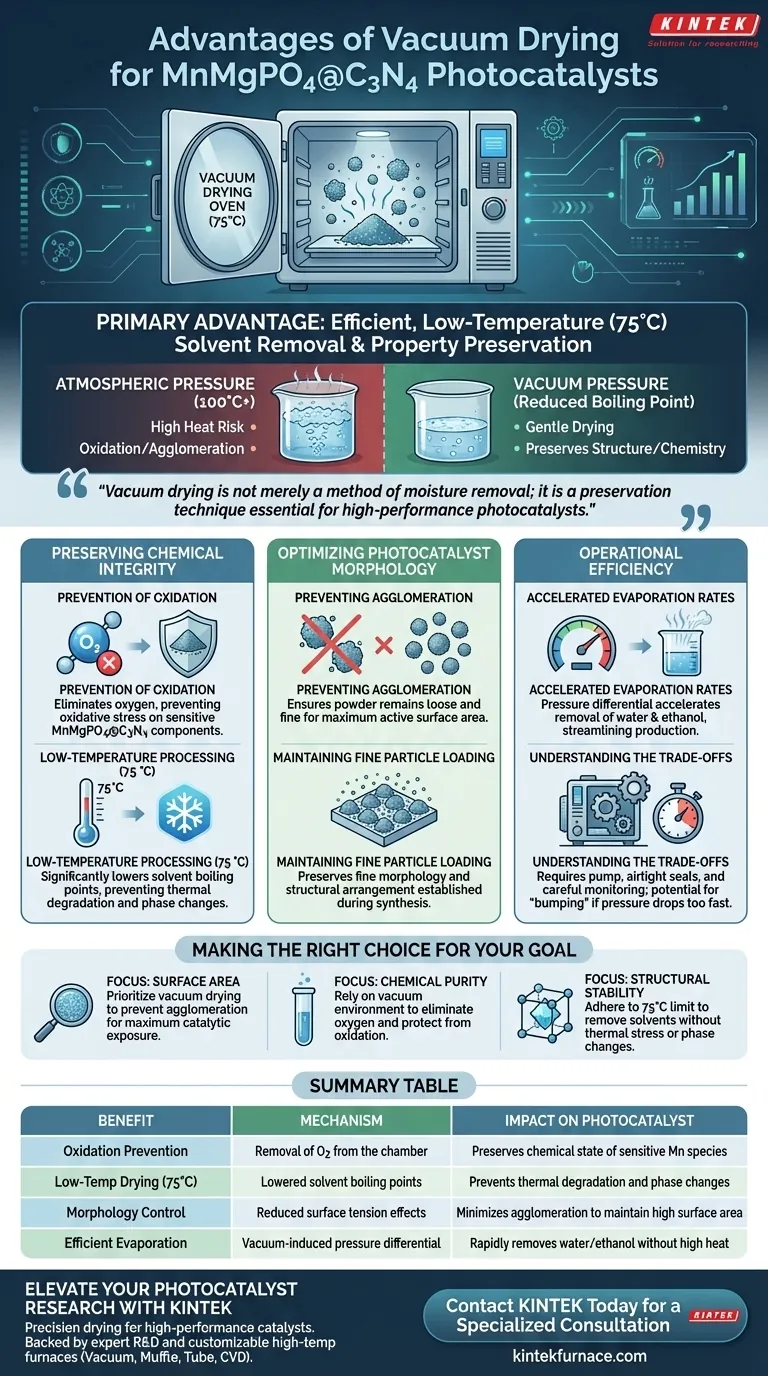
The primary advantage of using a vacuum drying oven for MnMgPO4@C3N4 photocatalyst powders is the ability to efficiently remove solvents like water and ethanol at a moderate temperature of 75 °C.
By lowering the environmental pressure, the oven reduces the boiling point of these liquids, accelerating their evaporation without subjecting the composite material to excessive heat. This method is specifically engineered to preserve the delicate structural and chemical properties of the photocatalyst.
Core Takeaway Vacuum drying is not merely a method of moisture removal; it is a preservation technique essential for high-performance photocatalysts. By decoupling drying speed from high heat, it prevents oxidation and agglomeration, ensuring the MnMgPO4 particles remain evenly distributed and chemically active on the C3N4 substrate.

Preserving Chemical Integrity
Prevention of Oxidation
The most critical chemical advantage of vacuum drying is the removal of oxygen from the drying environment.
MnMgPO4@C3N4 composites are sensitive to oxidative stress, which can alter the chemical state of the components. By drying under vacuum, you eliminate the risk of oxidation that frequently occurs in standard air-drying ovens.
Low-Temperature Processing
The vacuum environment significantly lowers the boiling point of solvents like water and ethanol.
This allows the material to be dried effectively at 75 °C, a temperature that is gentle enough to avoid thermal degradation. This protects the intrinsic properties of the material, preventing phase changes that might result from higher thermal loads.
Optimizing Photocatalyst Morphology
Preventing Agglomeration
For a photocatalyst to function effectively, it must maintain a high surface area.
Standard drying often causes particles to clump together as solvents evaporate, reducing the active surface area. Vacuum drying mitigates this "unwanted agglomeration," ensuring the resulting powder remains loose and fine.
Maintaining Fine Particle Loading
The effectiveness of the MnMgPO4@C3N4 composite relies on how well the MnMgPO4 particles are loaded onto the C3N4 surface.
The controlled drying process preserves the fine morphology of these particles. It ensures that the structural arrangement established during synthesis is not destroyed during the final drying step.
Operational Efficiency
Accelerated Evaporation Rates
Despite using a lower temperature, the process is efficient.
The vacuum environment creates a pressure differential that forces solvents to volatilize rapidly. This accelerates the removal of both water and ethanol, streamlining the production process without sacrificing material quality.
Understanding the Trade-offs
While vacuum drying is superior for quality, it introduces specific operational constraints compared to standard convection drying.
Equipment Complexity
Unlike a standard oven, a vacuum setup requires a pump and airtight seals. Any leak in the system can reintroduce oxygen or prevent the pressure from dropping low enough to facilitate low-temperature boiling.
Batch Limitations
Vacuum drying is typically a batch process that requires careful monitoring of pressure levels. If the pressure drops too suddenly, there is a risk of "bumping," where the solvent boils violently and splatters the fine powder, potentially leading to material loss.
Making the Right Choice for Your Goal
To maximize the performance of your MnMgPO4@C3N4 photocatalyst, apply the following guidelines:
- If your primary focus is Surface Area: Prioritize vacuum drying to prevent agglomeration, ensuring the powder remains loose for maximum catalytic exposure.
- If your primary focus is Chemical Purity: Rely on the vacuum environment to eliminate oxygen, protecting the manganese species and C3N4 structure from oxidation.
- If your primary focus is Structural Stability: adhere strictly to the 75 °C limit to remove water and ethanol without inducing thermal stress or phase changes.
The vacuum oven is the definitive tool for synthesizing photocatalysts where morphology and chemical state are as critical as dryness.
Summary Table:
| Benefit | Mechanism | Impact on Photocatalyst |
|---|---|---|
| Oxidation Prevention | Removal of O2 from the chamber | Preserves chemical state of sensitive Mn species |
| Low-Temp Drying (75°C) | Lowered solvent boiling points | Prevents thermal degradation and phase changes |
| Morphology Control | Reduced surface tension effects | Minimizes agglomeration to maintain high surface area |
| Efficient Evaporation | Vacuum-induced pressure differential | Rapidly removes water/ethanol without high heat |
Elevate Your Photocatalyst Research with KINTEK
Precision drying is the difference between a mediocre material and a high-performance catalyst. KINTEK provides industry-leading thermal solutions, including Vacuum, Muffle, Tube, and CVD systems, specifically engineered to protect delicate chemical structures like MnMgPO4@C3N4.
Backed by expert R&D and advanced manufacturing, our lab high-temp furnaces are fully customizable to meet your unique synthesis requirements. Don't compromise your material's morphology—leverage KINTEK's technical expertise to ensure chemical purity and structural stability in every batch.
Contact KINTEK Today for a Specialized Consultation
Visual Guide
References
- Ting Cheng, Fei Wu. Construction of Advanced S-Scheme Heterojunction Interface Composites of Bimetallic Phosphate MnMgPO4 with C3N4 Surface with Remarkable Performance in Photocatalytic Hydrogen Production and Pollutant Degradation. DOI: 10.3390/coatings15010103
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Vacuum Hot Press Furnace Machine Heated Vacuum Press Tube Furnace
- Vacuum Heat Treat Sintering and Brazing Furnace
- Vacuum Hot Press Furnace Machine for Lamination and Heating
People Also Ask
- What is the primary function of carbonization equipment? Master Biomass to Fuel Conversion with Precision
- Why is a sealed heating vessel used with a stepped heating process to infiltrate sulfur? Maximize Li-S Battery Performance
- What is the primary purpose of utilizing an argon gas purging process during waste pyrolysis? Ensure Pure Decomposition
- What are the characteristics of a Batch Reactor for plastic pyrolysis? A Guide to Versatile Waste Processing
- How does temperature control affect nanoporous copper dealloying? Master Pore Uniformity and Size
- How does a needle valve control silver foil surface quality for graphene growth? Prevent defects with pressure control.
- Why do high-performance Bi-2223 superconducting materials require high-precision temperature control? | KINTEK Solution
- What are the material and structural requirements for heating walls? Optimize Your Externally Heated Retorting Furnace



















