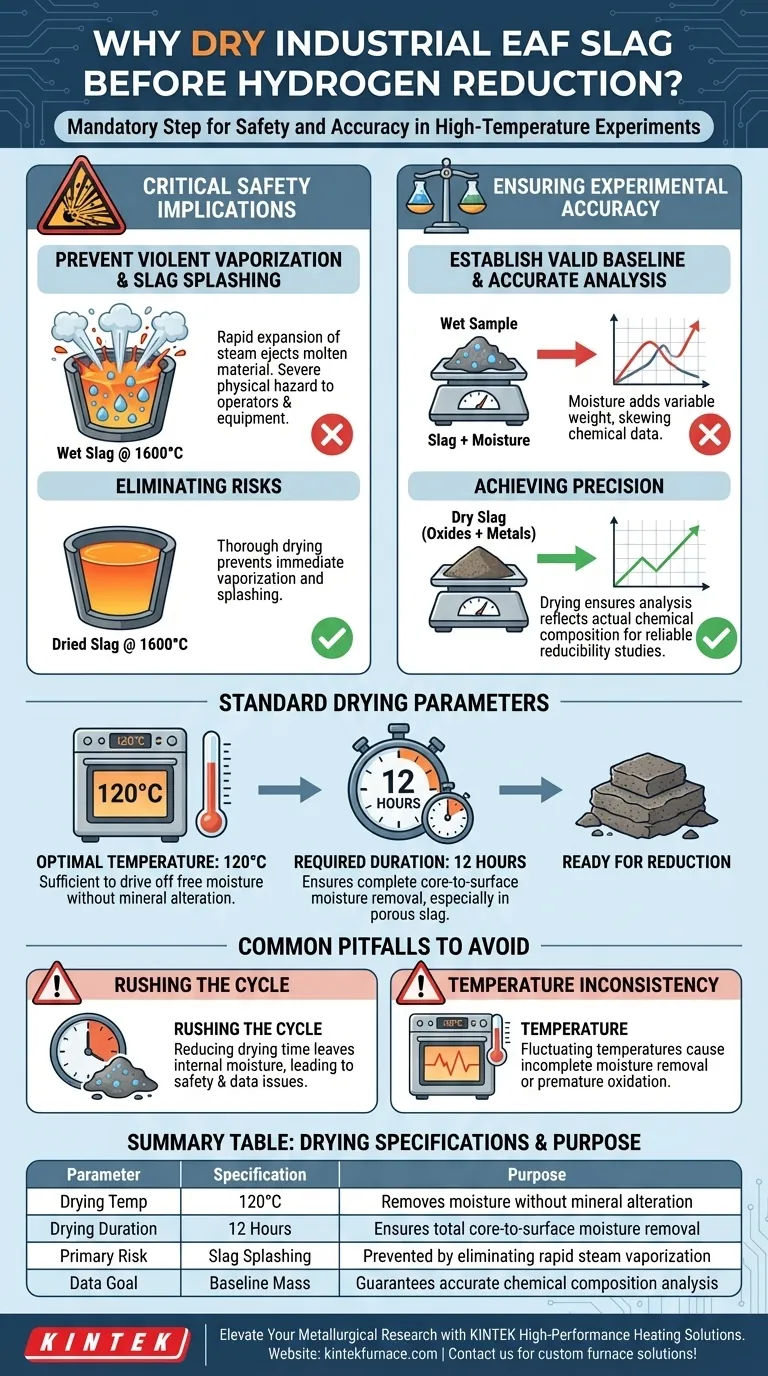
Drying Industrial Electric Arc Furnace (EAF) slag is a mandatory preparatory step required to eliminate residual moisture before high-temperature processing. By heating the raw material to 120°C for 12 hours, you ensure the integrity of chemical analysis and prevent dangerous physical reactions during the melting phase.
The removal of moisture is not merely a quality control measure; it is a fundamental safety requirement. Residual water in EAF slag can cause violent splashing at melting temperatures (1600°C) and skews the baseline data needed for accurate chemical composition analysis.

The Critical Safety Implications
Preventing Violent Vaporization
The most immediate risk in high-temperature experiments is the presence of water.
When slag containing moisture is introduced to a furnace operating at 1600°C, the water vaporizes instantly.
Eliminating Slag Splashing
This rapid expansion of steam creates a phenomenon known as slag splashing.
If the material is not thoroughly dried, the escaping steam can eject molten material from the crucible. This poses a severe physical hazard to both the equipment and the operators conducting the experiment.
Ensuring Experimental Accuracy
Establishing a Valid Baseline
Hydrogen reduction experiments rely on precise measurements of mass and chemical change.
Moisture adds variable weight to the sample that is not part of the slag's chemical structure.
Accurate Chemical Composition Analysis
To understand the reducibility of the slag, you must first know its exact starting composition.
Drying ensures that subsequent chemical analyses reflect the actual oxide and metallic content of the slag, rather than a sample diluted by water weight.
Standard Drying Parameters
Optimal Temperature
The industry standard for this preparation is 120°C.
This temperature is sufficient to drive off free moisture without altering the fundamental mineralogy of the slag before the reduction experiment begins.
Required Duration
The process requires a sustained duration of 12 hours.
EAF slag can be porous or dense; this extended time ensures that moisture is removed completely from the core of the material, not just the surface.
Common Pitfalls to Avoid
Rushing the Drying Cycle
A common mistake is reducing the drying time to speed up the experiment.
If the slag is removed before the full 12-hour cycle, internal moisture may remain trapped, leading to the safety and data issues described above.
Temperature Inconsistency
f the drying oven does not maintain a steady 120°C, moisture removal may be incomplete.
Lower temperatures may fail to evaporate water trapped in deep pores, while significantly higher temperatures could potentially induce premature oxidation or structural changes depending on the slag's specific mineralogy.
Making the Right Choice for Your Experiment
To ensure the validity of your hydrogen reduction experiments, you must treat drying as a critical control variable.
- If your primary focus is Safety: Strictly adhere to the drying protocol to prevent rapid vaporization and molten splashing at 1600°C.
- If your primary focus is Data Accuracy: Ensure the 12-hour cycle is completed to guarantee that mass balance calculations are based solely on the dry slag material.
Treat the drying phase not as a suggestion, but as a rigid prerequisite for valid and safe metallurgical research.
Summary Table:
| Parameter | Specification | Purpose |
|---|---|---|
| Drying Temperature | 120°C | Removes free moisture without mineral alteration |
| Drying Duration | 12 Hours | Ensures total core-to-surface moisture removal |
| Operating Temp | Up to 1600°C | Target melting point for reduction phase |
| Primary Risk | Slag Splashing | Prevented by eliminating rapid steam vaporization |
| Data Goal | Baseline Mass | Guarantees accurate chemical composition analysis |
Elevate Your Metallurgical Research with KINTEK
Precise slag drying and reduction experiments require high-performance heating solutions. Backed by expert R&D and manufacturing, KINTEK offers high-temperature Muffle, Tube, Rotary, Vacuum, and CVD systems designed to meet the rigorous demands of industrial metallurgy. Our customizable furnaces ensure the temperature consistency and safety needed for your most critical hydrogen reduction studies.
Ready to optimize your lab efficiency? Contact us today for a custom solution!
Visual Guide
References
- M. A. Levchenko, Olena Volkova. Reduction of Liquid Steelmaking Slag Using Hydrogen Gas as a Reductant. DOI: 10.3390/met15090984
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Electric Rotary Kiln Continuous Working Small Rotary Furnace Kiln for Pyrolysis Plant Heating
- Spark Plasma Sintering SPS Furnace
- 1400℃ Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- Why is a high-precision mass flow controller essential for long-term restart performance testing of catalysts?
- What is the chemical vapor transport technique? A Guide to High-Purity Crystal Growth
- How do magnesium impurities influence lithium extraction? Accelerate Production with Heterogeneous Nucleation
- What is the necessity of using a laboratory vacuum drying oven? Preserving Porous Carbon Integrity
- Why is a mixture of Argon (Ar) and Hydrogen (H2) required during beryl heat treatment? Master Color Transformation
- What are the advantages of using a vacuum drying oven for ZIF67/MXene? Protect Your Composite Integrity
- What function does a high-temperature furnace serve in alumina nanopowder decarbonization? Ensure Purity & Performance
- Why is a two-stage heat treatment required for Ca2Fe2O5? Optimize Your Brownmillerite Synthesis



















