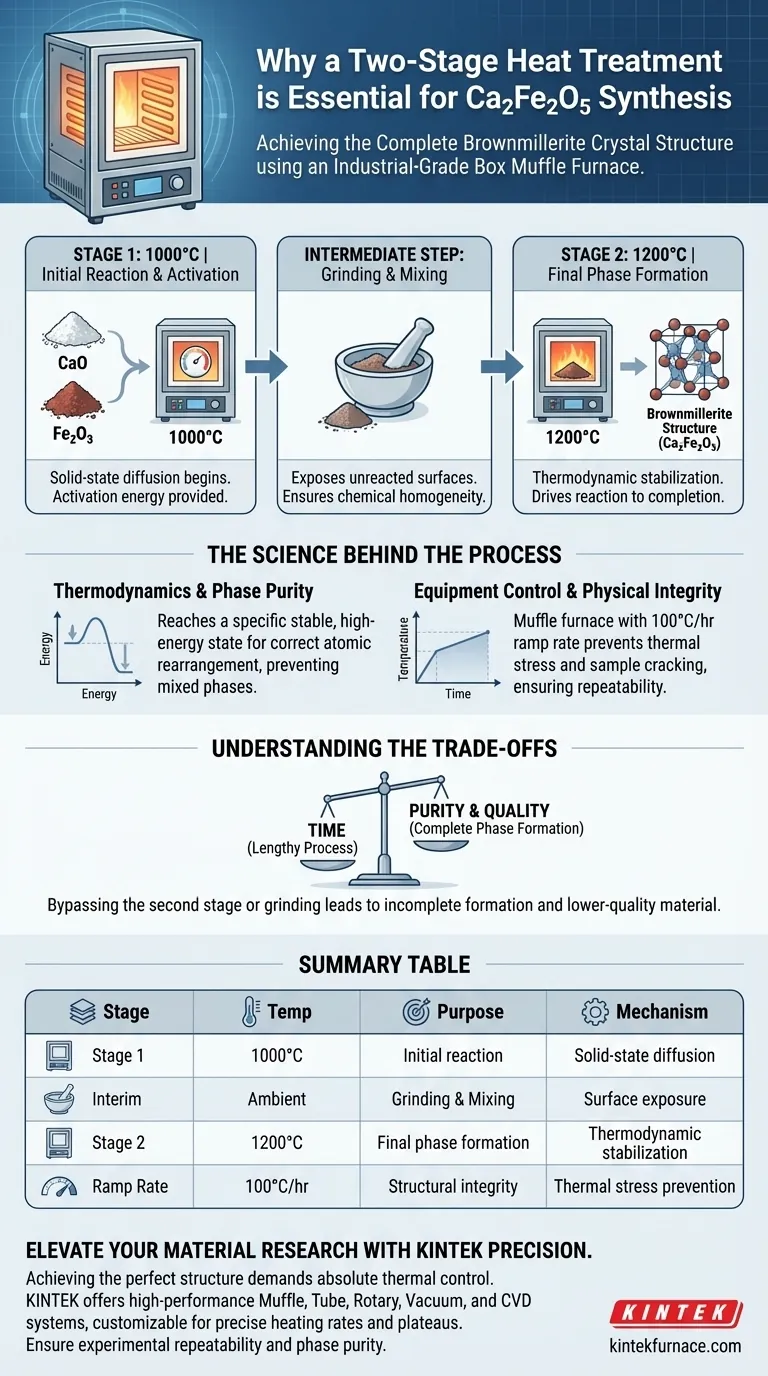
A two-stage heat treatment strategy is strictly required for the preparation of Ca2Fe2O5 to ensure the complete thermodynamic formation of its specific brownmillerite crystal structure. Utilizing an industrial-grade box muffle furnace allows for the necessary high-temperature plateaus—specifically at 1000°C and 1200°C—while an intermediate grinding step between these stages ensures full phase generation and chemical homogeneity.
The synthesis of Ca2Fe2O5 is a balance of thermodynamics and physical integrity. The two-stage firing process drives the chemical reaction to completion, while the precise control of the muffle furnace prevents structural failure due to thermal stress.

The Thermodynamics of Phase Formation
Achieving the Brownmillerite Structure
The creation of Ca2Fe2O5 is not merely about heating raw materials; it is about reaching a specific thermodynamic state.
The industrial-grade box muffle furnace provides the stable, high-energy environment required to form the brownmillerite structure.
The Necessity of High Temperatures
Specific temperature benchmarks are non-negotiable for this material.
The protocol requires dwell times at 1000°C and 1200°C. These temperatures provide the activation energy necessary to rearrange the atomic structure into the correct crystalline phase.
Overcoming Reaction Limitations
The Role of Intermediate Grinding
Heating alone is often insufficient for solid-state reactions due to limited particle contact.
The two-stage process includes intermediate grinding between the firing cycles.
This mechanical step exposes unreacted surfaces and mixes the material, ensuring complete phase generation rather than a mixture of reacted and unreacted powder.
Ensuring Homogeneity
Without the interruption to grind and mix the sample, the reaction might stall.
The two-stage approach guarantees that the final product is chemically uniform throughout the sample volume.
The Critical Role of Equipment Control
Precision Heating Profiles
An industrial-grade muffle furnace is required because it offers adjustable heating rates, which are critical for sample survival.
The standard protocol typically utilizes a ramp rate of 100°C per hour.
Preventing Physical Failure
Rapid heating in less sophisticated equipment often leads to failure.
Controlled heating prevents sample cracking caused by thermal stress.
By managing the thermal expansion slowly, the furnace ensures the physical integrity of the samples and guarantees experimental repeatability.
Understanding the Trade-offs
Time vs. Purity
The primary trade-off in this two-stage method is time.
Heating to 1200°C at a rate of 100°C per hour, combined with a cooling and grinding phase, creates a lengthy synthesis process.
However, attempting to bypass the second stage or the intermediate grinding invariably leads to incomplete phase formation and lower-quality material.
Making the Right Choice for Your Goal
To ensure the successful synthesis of Ca2Fe2O5, apply the following principles based on your specific requirements:
- If your primary focus is phase purity: Strictly adhere to the intermediate grinding step between the 1000°C and 1200°C firings to drive the reaction to completion.
- If your primary focus is physical integrity: Do not exceed the heating rate of 100°C per hour to avoid thermal stress fractures in the sample.
Precision in the thermal profile is just as critical as the chemistry itself for achieving a high-quality brownmillerite structure.
Summary Table:
| Stage | Temperature | Purpose | Key Mechanism |
|---|---|---|---|
| Stage 1 | 1000°C | Initial reaction & activation | Solid-state diffusion |
| Interim | Ambient | Intermediate grinding | Surface exposure & mixing |
| Stage 2 | 1200°C | Final phase formation | Thermodynamic stabilization |
| Ramp Rate | 100°C/hr | Structural integrity | Thermal stress prevention |
Elevate Your Material Research with KINTEK Precision
Achieving the perfect brownmillerite structure demands absolute thermal control. Backed by expert R&D and manufacturing, KINTEK offers high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems—all customizable to meet your specific lab requirements. Whether you are synthesizing Ca2Fe2O5 or developing advanced ceramics, our industrial-grade furnaces ensure the precise heating rates and temperature plateaus necessary for experimental repeatability and phase purity.
Ready to optimize your synthesis process? Contact our laboratory specialists today to find the ideal high-temperature solution for your unique needs!
Visual Guide
References
- E. Schultz, Ram Krishna Hona. Thermoelectric Effect of Ca<sub>2</sub>Fe<sub>2</sub>O<sub>5</sub> at Low Temperatures. DOI: 10.4236/msce.2025.136001
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What is the importance of a stable thermal environment during crystallization? Ensure Precision in Metal Oxide Films
- Why is an 800 °C heat treatment for Ti6Al4V additive manufacturing necessary? Unlock Ductility & Relieve Stress
- What are the benefits of using a vacuum environment for RCM NSs? Master Material Preservation & Catalytic Performance
- Why is vacuum degassing necessary for ZIF-8 impregnation? Achieve Uniform Macroporous Material Synthesis
- Why is high-precision constant temperature heating equipment required when preparing 17-4 PH stainless steel composite?
- What is the main purpose of heat treatment? Transform Metal Properties for Superior Performance
- What is the purpose of using generalized governing equations? | Expert 3D Unsteady Furnace Modeling
- What gas is used in a graphite furnace? A Guide to Argon vs. Nitrogen for Optimal Analysis



















