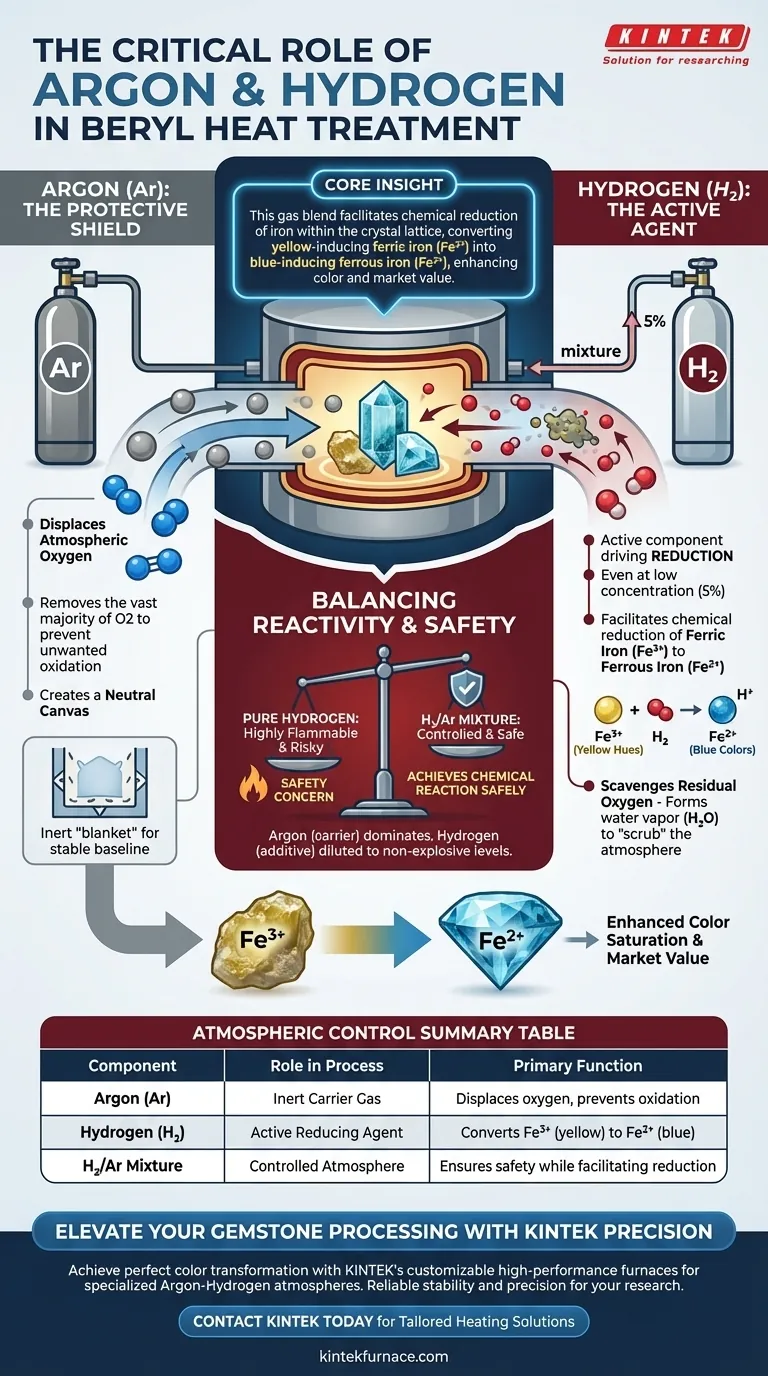
The mixture of Argon (Ar) and Hydrogen (H2) performs a critical dual function essential for altering the color of beryl gemstones. Argon serves as an inert "shield" to displace atmospheric oxygen, while Hydrogen acts as the active reducing agent that chemically alters the stone's impurities. This combination creates a controlled environment where oxidation is prevented, and favorable color transformation is induced.
Core Insight This gas blend is strictly required to facilitate the chemical reduction of iron within the crystal lattice. By converting yellow-inducing ferric iron ($Fe^{3+}$) into blue-inducing ferrous iron ($Fe^{2+}$), the treatment permanently enhances the beryl's color saturation and market value.

The Role of Argon: The Protective Shield
Displacing Atmospheric Oxygen
Argon is an inert noble gas, meaning it does not chemically react with the gemstone. Its primary purpose in this process is to act as a protective volume.
By flooding the furnace chamber with Argon, you physically displace the ambient air. This removes the vast majority of oxygen, which is necessary to prevent unwanted oxidation of the gemstone or the furnace components.
Creating a Neutral Canvas
Argon creates a stable, neutral baseline for the heat treatment. Without this inert "blanket," the reactive gases introduced later would interact unpredictably with atmospheric elements.
The Role of Hydrogen: The Active Agent
The Reduction Mechanism
Hydrogen is the active component that drives the specific desired result: reduction. Even at a low concentration (typically 5%), Hydrogen acts as a potent reducing agent.
The heat treatment targets iron impurities within the beryl. Hydrogen facilitates the chemical reduction of Ferric iron ($Fe^{3+}$), which causes yellow hues, into Ferrous iron ($Fe^{2+}$).
Enhancing Color Saturation
This chemical shift is the "Why" behind the process. The conversion to $Fe^{2+}$ is responsible for producing the desirable blue colors often sought in aquamarine and other beryl varieties.
Scavenging Residual Oxygen
While Argon displaces most air, trace amounts of oxygen may remain. Hydrogen reacts with this residual oxygen to form water vapor, effectively "scrubbing" the atmosphere to ensure a pure reduction environment.
Understanding the Trade-offs
Balancing Reactivity and Safety
You might ask why pure Hydrogen isn't used. Pure Hydrogen is highly flammable and presents significant safety risks in high-temperature furnaces.
By using a mixture where Argon is the dominant carrier and Hydrogen is a minor additive (5%), you achieve the necessary chemical reaction without the volatility of a pure hydrogen atmosphere.
Necessity of the Mixture
Using Argon alone would prevent oxidation, but it would not improve the color, as no reducing agent would be present to convert the iron. Conversely, an oxygen-rich environment would prohibit the reduction entirely. Therefore, the specific mixture is non-negotiable for color improvement.
Making the Right Choice for Your Goal
To achieve the desired aesthetic results in beryl heat treatment, you must control the atmosphere based on the specific iron transformation required.
- If your primary focus is Eliminating Yellow Hues: You must introduce Hydrogen to successfully reduce $Fe^{3+}$ ions to $Fe^{2+}$.
- If your primary focus is Process Safety: Rely on Argon as the bulk carrier gas to maintain positive pressure and dilute the flammable Hydrogen to non-explosive levels.
Precise atmospheric control is the only way to reliably unlock the coveted blue tones hidden within the gemstone's structure.
Summary Table:
| Component | Role in Process | Primary Function |
|---|---|---|
| Argon (Ar) | Inert Carrier Gas | Displaces oxygen and prevents unwanted oxidation |
| Hydrogen (H2) | Active Reducing Agent | Converts $Fe^{3+}$ (yellow) to $Fe^{2+}$ (blue) |
| H2/Ar Mixture | Controlled Atmosphere | Ensures safety while facilitating chemical reduction |
Elevate Your Gemstone Processing with KINTEK Precision
Achieving the perfect color transformation in beryl requires absolute control over your thermal environment. Backed by expert R&D and manufacturing, KINTEK offers high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems—all fully customizable to handle specialized Argon-Hydrogen atmospheres.
Whether you are looking to eliminate yellow hues or maximize process safety, our laboratory high-temperature furnaces provide the stability and precision your research demands. Contact KINTEK today to discover how our tailored heating solutions can enhance your lab's efficiency and output.
Visual Guide
References
- Bin Hao, Qingfeng Guo. The Effect of Heat Treatment on Yellow-Green Beryl Color and Its Enhancement Mechanism. DOI: 10.3390/cryst15080746
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
People Also Ask
- What key process environments does a Molecular Beam Epitaxy (MBE) system provide? Optimize CaF2 Thin Film Growth
- What is Skin Depth and how does it affect induction heating? Master Frequency Control for Precise Heat
- What is the primary function of a high-precision program-controlled furnace? Mastering T6 Heat Treatment of Al-Cu 224
- What is the graphite furnace technique? A Guide to Ultra-Trace Metal Analysis
- What are the benefits of ESR for carbonitride distribution in H13 steel? Enhance Your Material's Isotropic Properties
- How does a single-roller melt-spinning system facilitate Fe-based amorphous alloys? Achieve Precision Rapid Quenching
- Why is a 1200°C hold required for Ti(C,N)-FeCr sintering? Unlock Superior Material Density
- Why is 600 °C critical for ZIF-8 carbonization? Achieve Optimal Surface Area and Functional Group Retention



















