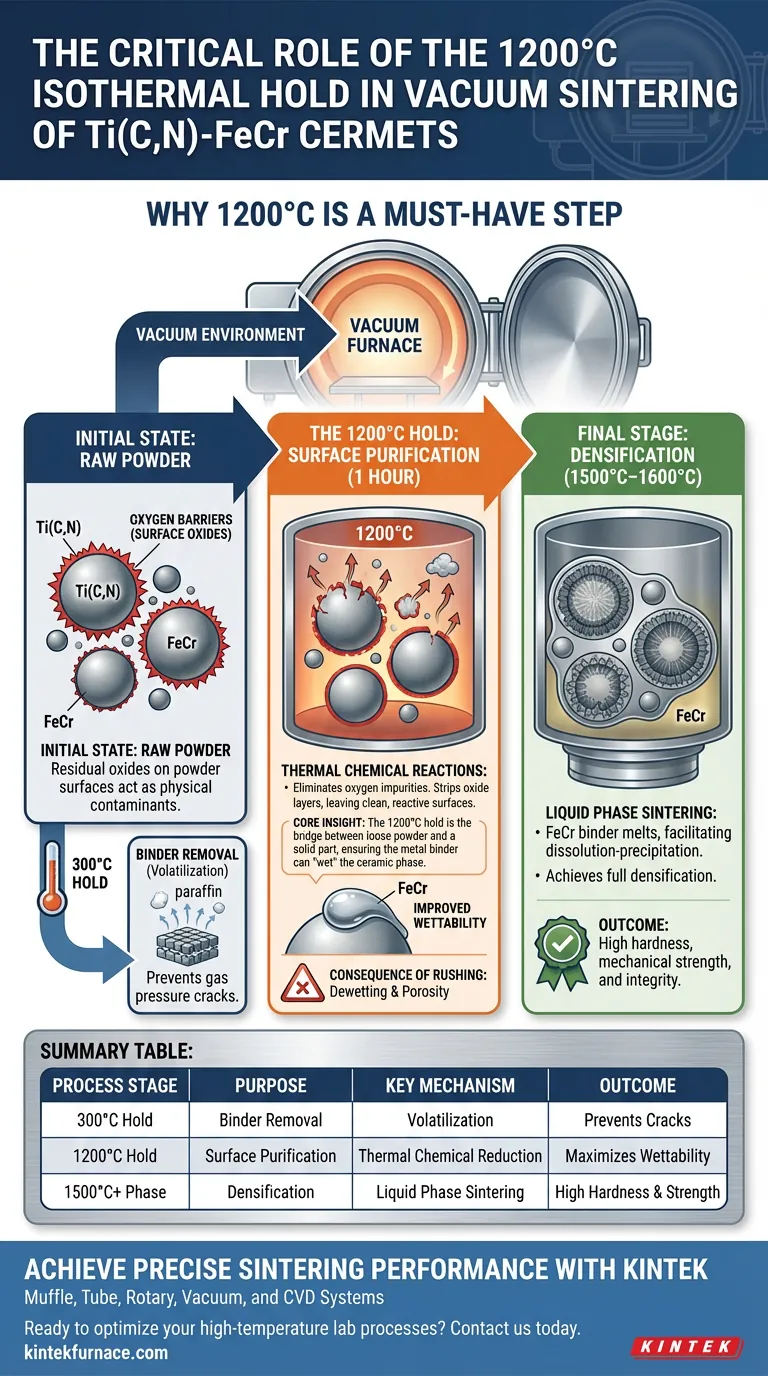
A long-duration isothermal hold at 1200°C is strictly necessary to chemically purify the material surfaces before melting occurs. Specifically, this one-hour phase in the vacuum sintering furnace is designed to fully reduce residual oxides found on the powder particles. By utilizing the vacuum environment to facilitate thermal chemical reactions, this step eliminates oxygen impurities that act as barriers to bonding.
Core Insight: The 1200°C hold is the bridge between loose powder and a solid part. By stripping away surface oxides, it ensures the metal binder can "wet" the ceramic phase. Without this chemical preparation, the subsequent liquid phase sintering will fail to achieve full densification.
The Critical Role of Surface Chemistry
Eliminating Oxygen Barriers
The primary obstacle to high-quality cermets is surface oxidation.
Raw powders used in Ti(C,N)-FeCr cermets inevitably contain residual oxides on their surfaces. If these oxides remain during the high-temperature phases, they act as a physical contaminant that prevents the materials from fusing.
The Mechanism of Reduction
The 1200°C isothermal hold triggers thermal chemical reactions within the vacuum environment.
Maintaining this temperature for one hour provides sufficient time and energy to break down these oxide layers. This process effectively strips oxygen impurities from the system, leaving behind clean, reactive surfaces on the powder particles.
From Purification to Densification
Improving Wettability
The immediate result of oxide removal is significantly improved wettability.
Wettability refers to the ability of the liquid metal binder (FeCr) to spread over and adhere to the solid ceramic phase (Ti(C,N)). A clean, oxide-free surface is the only surface the binder can effectively "wet."
Enabling Liquid Phase Sintering
This wettability is the necessary prerequisite for the final stage of the process.
Once the furnace ramps up to 1500°C–1600°C, the FeCr binder melts to trigger liquid phase sintering. Because the 1200°C step removed the oxides, the binder can now facilitate the dissolution-precipitation reactions required to form the complex "core-rim" microstructure, ensuring full densification.
Understanding the Process Risks
The Consequence of Rushing
Reducing the duration of the 1200°C hold is a critical error in process design.
If the hold is too short, residual oxides will remain. This leads to "dewetting," where the binder beads up rather than spreading. The final result is a material with high porosity, poor mechanical bonding, and compromised hardness.
Distinguishing Process Stages
It is vital not to confuse the purpose of the 1200°C hold with the 300°C hold.
While the 1200°C hold manages chemical purity (oxides), the 300°C hold manages structural integrity by slowly removing forming agents like paraffin. Neglecting the lower temperature hold causes gas pressure cracks; neglecting the 1200°C hold causes metallurgical failure.
Making the Right Choice for Your Goal
To achieve specific material properties, you must optimize each stage of the vacuum sintering cycle:
- If your primary focus is Maximum Density: Ensure the 1200°C hold is maintained for the full hour to guarantee complete oxide reduction and optimal binder wettability.
- If your primary focus is Structural Integrity: Do not overlook the 300°C hold; precise temperature control here prevents rapid volatilization of forming agents that leads to cracking.
- If your primary focus is Hardness and Toughness: rely on the 1500°C–1600°C stage to form the rim phases, but remember this is impossible without the surface preparation done at 1200°C.
Success in sintering Ti(C,N)-FeCr cermets relies on a clean surface just as much as high heat; the 1200°C hold is the gatekeeper of that cleanliness.
Summary Table:
| Process Stage | Purpose | Key Mechanism | Outcome |
|---|---|---|---|
| 300°C Hold | Binder Removal | Volatilization of forming agents (paraffin) | Prevents gas pressure cracks |
| 1200°C Hold | Surface Purification | Thermal chemical reduction of oxides | Maximizes wettability for the binder |
| 1500°C+ Phase | Densification | Liquid phase sintering & core-rim formation | High hardness and mechanical strength |
Achieve Precise Sintering Performance with KINTEK
Don't let surface impurities compromise your material integrity. KINTEK provides industry-leading vacuum sintering solutions backed by expert R&D and manufacturing. Our Muffle, Tube, Rotary, Vacuum, and CVD systems are fully customizable to meet the rigorous demands of Ti(C,N)-FeCr cermet production, ensuring precise temperature holds and superior vacuum environments.
Ready to optimize your high-temperature lab processes? Contact us today to discover how our tailor-made furnace solutions can enhance your densification and material quality.
Visual Guide
References
- T.H. Pampori, Jakob Kübarsepp. Exploring Microstructural Properties, Phase Transformations, and Wettability in High-Chromium Content Iron-bonded Ti(C,N)-based Cermet. DOI: 10.2497/jjspm.16p-t14-06
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Molybdenum Vacuum Heat Treat Furnace
- Vacuum Heat Treat Sintering Furnace Molybdenum Wire Vacuum Sintering Furnace
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
People Also Ask
- Why is a glove box necessary for aluminum foil pre-lithiation? Ensure Purity in Anode Development
- Why is a two-stage heat treatment required for Ca2Fe2O5? Optimize Your Brownmillerite Synthesis
- What is the role of a 5% N2 + 95% H2 mixture in plasma nitriding? Enhance Fatigue Strength and Eliminate White Layers
- What is the importance of a laboratory oven's programmed heating for epoxy-polyimide curing? Essential Thermal Control
- What are the advantages of using the DO radiation model in high-temp furnaces? Boost Precision & Emission Control
- What is the significance of using a hydrogen etching process in a reaction chamber? Mastering SiC Surface Preparation
- How does the thermal field length impact YAG fiber dip coatings? Achieve Uniform, Bead-Free Films
- What is the role of MgO powder in Nickel-Aluminum VCS? Achieve Precise Thermal Control & Powder Quality



















