At its core, the graphite furnace technique is a highly sensitive method used in analytical chemistry to measure the concentration of specific elements within a sample. Formally known as Graphite Furnace Atomic Absorption Spectrometry (GFAAS), it uses an electrically heated graphite tube to vaporize a minuscule amount of sample, creating a cloud of free atoms that can be measured with extreme precision.
The graphite furnace technique is not just another heating method; it is a highly controlled electrothermal atomization process for Atomic Absorption Spectrometry (AAS). It excels at detecting trace and ultra-trace metal concentrations by vaporizing a tiny sample in an inert atmosphere, offering sensitivity far superior to traditional flame-based methods.

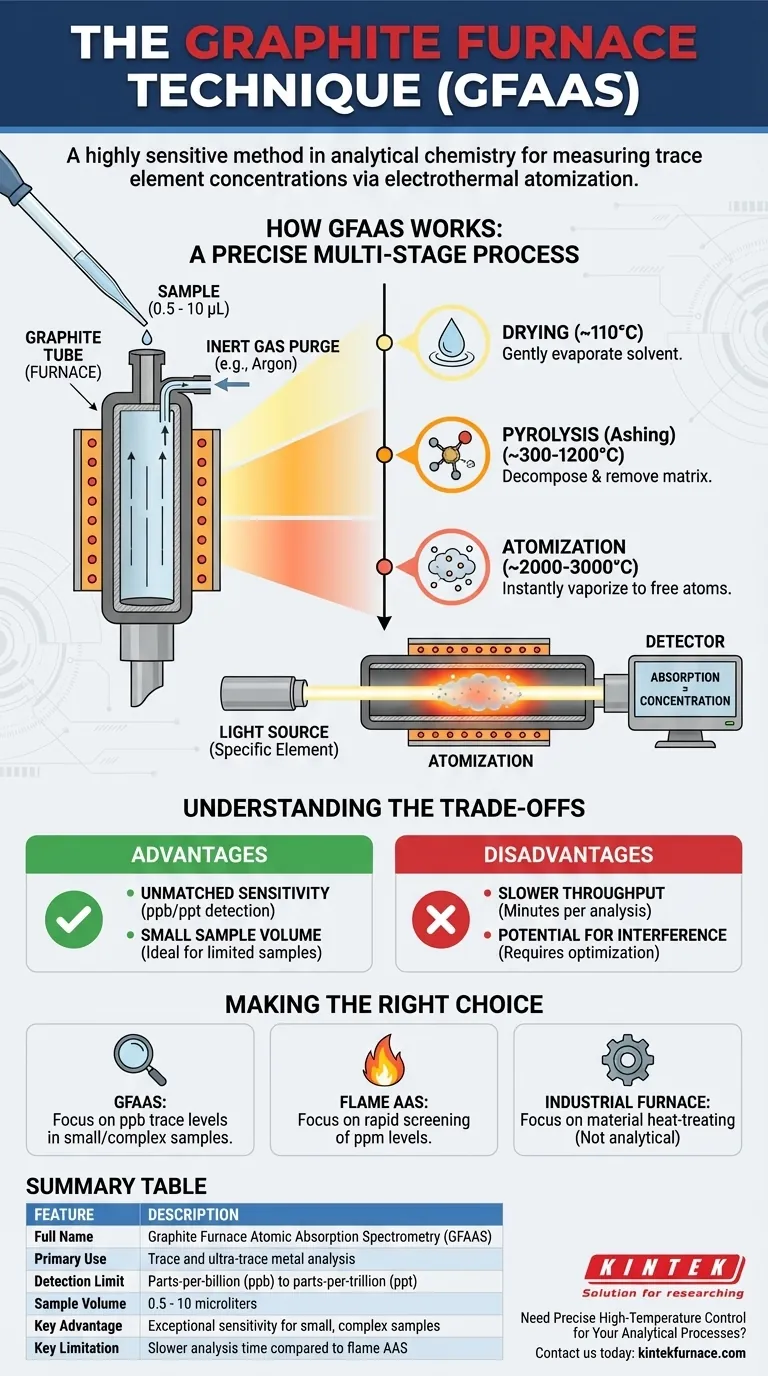
How the Graphite Furnace Technique Works
The power of the technique lies in its precise, multi-stage heating process performed inside a small, controlled environment. This allows for the complete atomization of the target element while minimizing interferences.
The Core Component: The Graphite Tube
The heart of the system is a small tube made of high-purity graphite. This tube acts as a miniature furnace, heating up rapidly when an electric current is passed through it.
A very small sample volume, typically between 0.5 and 10 microliters, is injected into the tube through a small hole. The entire furnace is sealed and purged with an inert gas, usually argon, to prevent the hot graphite from combusting and to remove atmospheric oxygen that could interfere with the analysis.
The Three-Stage Heating Program
Unlike a simple oven, the graphite furnace follows a carefully programmed temperature sequence to isolate the element of interest.
- Drying: The temperature is raised gently (e.g., to 110 °C) to slowly evaporate the solvent from the sample droplet without sputtering.
- Pyrolysis (Ashing): The temperature is increased significantly (e.g., 300-1200 °C) to thermally decompose and remove the bulk of the sample matrix (organic matter, salts), leaving behind the more thermally stable analyte.
- Atomization: The temperature is rapidly ramped to a very high level (e.g., 2000-3000 °C). This intense heat instantly vaporizes the remaining residue, converting the analyte into a dense cloud of free, neutral atoms.
Measurement and Detection
During the final atomization step, a beam of light specific to the element being measured is passed through the graphite tube. The free atoms in the vapor cloud absorb a portion of this light.
A detector on the other side of the tube measures the decrease in light intensity. The amount of light absorbed is directly proportional to the concentration of the element in the original sample.
Understanding the Trade-offs
While powerful, GFAAS is not the right tool for every situation. Understanding its strengths and weaknesses is key to using it effectively.
Advantage: Unmatched Sensitivity
The primary reason to use GFAAS is its exceptional sensitivity. By concentrating the entire atomized sample in a small, confined space, it can achieve detection limits in the parts-per-billion (ppb) or even parts-per-trillion (ppt) range. This is often 100 to 1,000 times more sensitive than flame-based AAS.
Advantage: Small Sample Volume
The ability to work with microliter-sized samples is a major benefit when the sample is precious, limited, or difficult to obtain, such as in clinical or forensic applications.
Disadvantage: Slower Throughput
The multi-stage heating program means a single analysis can take several minutes. This makes GFAAS much slower than flame AAS, which can analyze samples almost continuously. It is not well-suited for high-volume screening.
Disadvantage: Potential for Interference
The high sensitivity of GFAAS also makes it more susceptible to chemical and spectral interferences from the sample matrix. Developing a robust method often requires careful optimization of the heating program and the use of chemical modifiers or advanced background correction systems.
Making the Right Choice for Your Application
Selecting the correct analytical technique depends entirely on your measurement goals.
- If your primary focus is detecting parts-per-billion (ppb) concentrations of metals in a small or complex sample: The graphite furnace technique (GFAAS) is the ideal choice due to its superior sensitivity.
- If your primary focus is rapidly screening many samples for higher, parts-per-million (ppm) concentrations: A traditional Flame Atomic Absorption Spectrometry (FAAS) system is a more efficient and cost-effective solution.
- If your primary focus is heat-treating industrial materials like steel or titanium components: You need an industrial vacuum furnace made of graphite, which is a materials-processing tool, not an analytical instrument.
Ultimately, choosing GFAAS is a strategic decision to prioritize sensitivity and precision for trace element analysis above all else.
Summary Table:
| Feature | Description |
|---|---|
| Full Name | Graphite Furnace Atomic Absorption Spectrometry (GFAAS) |
| Primary Use | Trace and ultra-trace metal analysis |
| Detection Limit | Parts-per-billion (ppb) to parts-per-trillion (ppt) |
| Sample Volume | 0.5 - 10 microliters |
| Key Advantage | Exceptional sensitivity for small, complex samples |
| Key Limitation | Slower analysis time compared to flame AAS |
Need Precise High-Temperature Control for Your Analytical Processes?
Leveraging exceptional R&D and in-house manufacturing, KINTEK provides diverse laboratories with advanced high-temperature furnace solutions. Our product line, including Muffle, Tube, Rotary Furnaces, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems, is complemented by our strong deep customization capability to precisely meet unique experimental requirements.
Contact us today to discuss how our robust and reliable furnace technology can enhance the precision and sensitivity of your analytical methods like GFAAS.
Visual Guide
Related Products
- Laboratory Quartz Tube Furnace RTP Heating Tubular Furnace
- Vertical Laboratory Quartz Tube Furnace Tubular Furnace
- Inclined Rotary Plasma Enhanced Chemical Deposition PECVD Tube Furnace Machine
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- Spark Plasma Sintering SPS Furnace
People Also Ask
- What factors should be considered when purchasing a quartz tube furnace? Ensure Reliable High-Temperature Processing
- What is a Quartz Tube Furnace and what is its primary function? Essential for Real-Time Material Observation
- What technical requirements affect the external thermal strength of furnace tubes? Optimize for High-Temp Performance
- How does the work process of a quartz tube furnace typically proceed? Master Precision Heating for Advanced Materials
- What are the key features of a quartz tube furnace? Discover High-Temp Precision for Your Lab



















