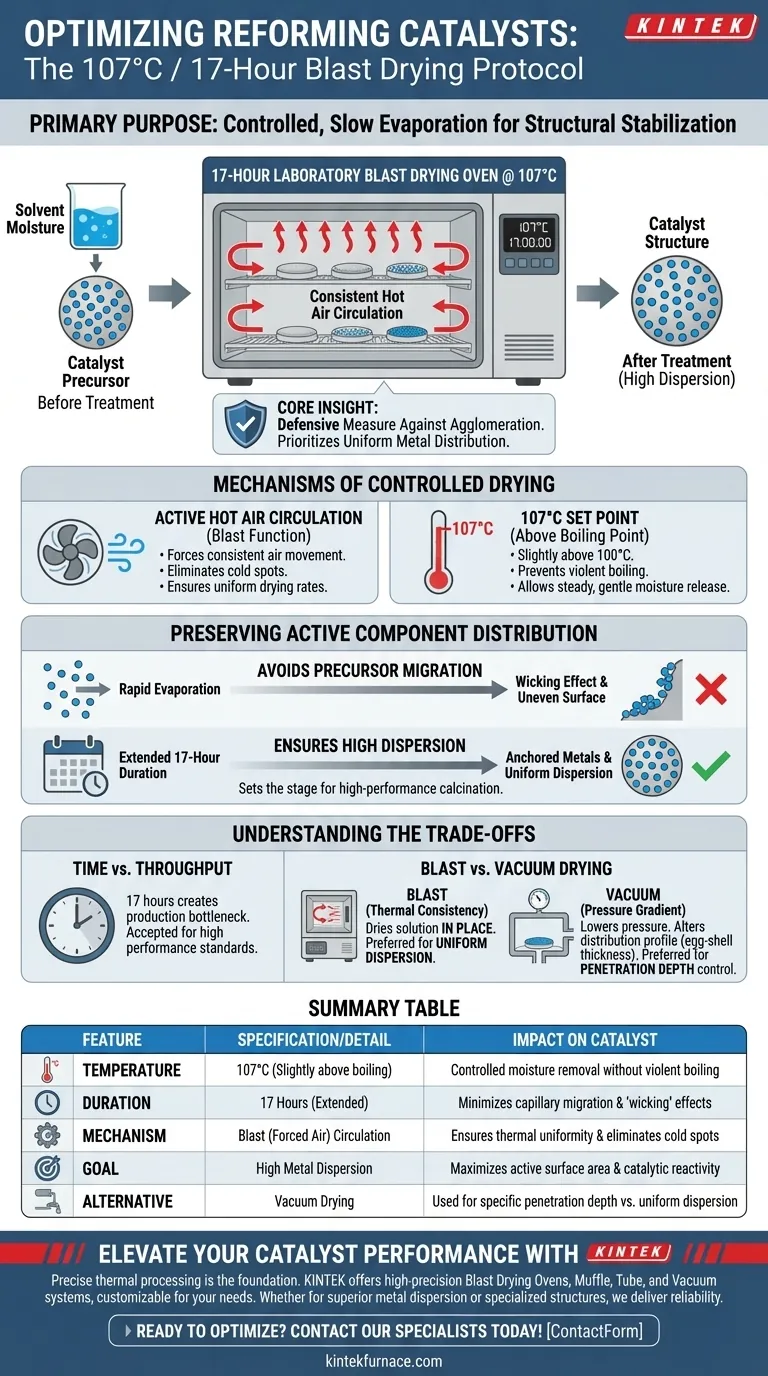
The primary purpose of this thermal treatment is to ensure the controlled, slow evaporation of solvent moisture from the loaded active metal nitrate precursors. By maintaining a temperature of 107°C with consistent hot air circulation for an extended 17-hour duration, the process stabilizes the catalyst structure before high-temperature calcination.
Core Insight: This extended drying protocol is a defensive measure against component agglomeration. It prioritizes the uniform distribution of active metals over processing speed, ensuring that the precursors do not migrate unevenly or clump together on the carbon support surface.

The Mechanisms of Controlled Drying
The Role of Hot Air Circulation
A laboratory blast drying oven distinguishes itself through active air circulation.
Unlike static ovens, the "blast" function forces hot air to move consistently around the sample. This ensures that the temperature remains uniform throughout the chamber, eliminating cold spots that could lead to uneven drying rates across the catalyst batch.
Significance of the 107°C Set Point
The specific temperature of 107°C is strategically chosen to be slightly above the boiling point of water.
This allows for the complete removal of the solvent moisture without inducing violent boiling or rapid vaporization. Rapid boiling can disrupt the pore structure or physically displace the metal precursors, whereas this temperature promotes a steady, gentle release of moisture.
Preserving Active Component Distribution
Preventing Precursor Migration
The most critical risk during the drying phase is the non-uniform migration of precursors.
If the solvent evaporates too quickly or unevenly, capillary forces can drag the dissolved metal nitrates toward the outer surface of the support. The 17-hour duration at a moderate temperature minimizes this "wicking" effect, keeping the metals anchored where they were originally deposited.
Ensuring High Dispersion
The ultimate goal of this step is to set the stage for the subsequent calcination process.
By preventing the precursors from agglomerating (clumping) during the drying phase, the method ensures high dispersion of the active components. High dispersion translates directly to a larger active surface area, which is essential for the catalyst's final reactivity and efficiency.
Understanding the Trade-offs
Time vs. Throughput
The most obvious trade-off of this method is the time investment. Dedicating 17 hours to a single drying step creates a bottleneck in production throughput, but this "cost" is generally accepted as necessary to achieve high-performance dispersion standards.
Blast Drying vs. Vacuum Drying
It is helpful to understand why a blast oven is used rather than a vacuum oven.
A vacuum drying oven lowers pressure to reduce the solvent's boiling point, creating a pressure gradient that can extract solution from internal pores. While this can mitigate deep penetration, it often results in a different distribution profile (intermediate egg-shell thickness).
The blast drying oven, conversely, does not rely on pressure gradients to pull fluid out. Instead, it relies on thermal consistency to dry the solution in place, which is generally preferred when uniform dispersion throughout the support is the priority.
Making the Right Choice for Your Protocol
Depending on the specific requirements of your reforming catalyst, verify that your drying method aligns with your structural goals.
- If your primary focus is high metal dispersion: Adhere strictly to the blast drying method (107°C for 17 hours) to prevent agglomeration and precursor migration.
- If your primary focus is controlling penetration depth: Investigate vacuum drying options, as the pressure gradient may help you manipulate how deep the solution remains within the pores.
Precision in the drying phase is the invisible foundation of a high-performance catalyst.
Summary Table:
| Feature | Specification/Detail | Impact on Catalyst |
|---|---|---|
| Temperature | 107°C (Slightly above boiling) | Controlled moisture removal without violent boiling |
| Duration | 17 Hours (Extended) | Minimizes capillary migration and "wicking" effects |
| Mechanism | Blast (Forced Air) Circulation | Ensures thermal uniformity and eliminates cold spots |
| Goal | High Metal Dispersion | Maximizes active surface area and catalytic reactivity |
| Alternative | Vacuum Drying | Used for specific penetration depth vs. uniform dispersion |
Elevate Your Catalyst Performance with KINTEK
Precise thermal processing is the foundation of high-performance catalyst synthesis. At KINTEK, we understand that maintaining exact temperature uniformity and controlled airflow is non-negotiable for your R&D and production success.
Backed by expert R&D and manufacturing, KINTEK offers high-precision Laboratory Blast Drying Ovens, Muffle, Tube, and Vacuum systems, all customizable to meet your unique chemical processing needs. Whether you are aiming for superior metal dispersion or specialized pore structures, our equipment delivers the reliability you demand.
Ready to optimize your drying protocol? Contact our specialists today to find the perfect thermal solution for your lab!
Visual Guide
References
- Soohyun Kim, Jeonghwan Lim. Steam Reforming of High-Concentration Toluene as a Model Biomass Tar Using a Nickel Catalyst Supported on Carbon Black. DOI: 10.3390/en18020327
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1200℃ Muffle Oven Furnace for Laboratory
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
People Also Ask
- Why is a nitrogen protection system necessary for LPF resin synthesis? Ensure Purity in Lab Polymerization
- What role does a forced air drying oven play in the preparation of zinc oxide nanoparticles? Prevent Agglomeration
- Why is a 105 °C drying process in an electric drying oven significant? Prevent Refractory Structural Failure
- Why is high-purity iodine utilized as a transport agent in the growth of MoS2 and MoSe2? Master CVT Crystal Growth
- What are the technical advantages of using a six-zone resistance heating furnace in VGF-VB? Unlock Precision Growth
- What is the purpose of an industrial oven for powder pre-treatment? Ensure Accurate Silica Analysis
- What role does active carbon play in CaS:Eu2+ phosphor synthesis? Key to Activating High-Efficiency Luminescence
- What is a crucible furnace used for? Achieve Pure, Controlled Melts for Non-Ferrous Metals



















