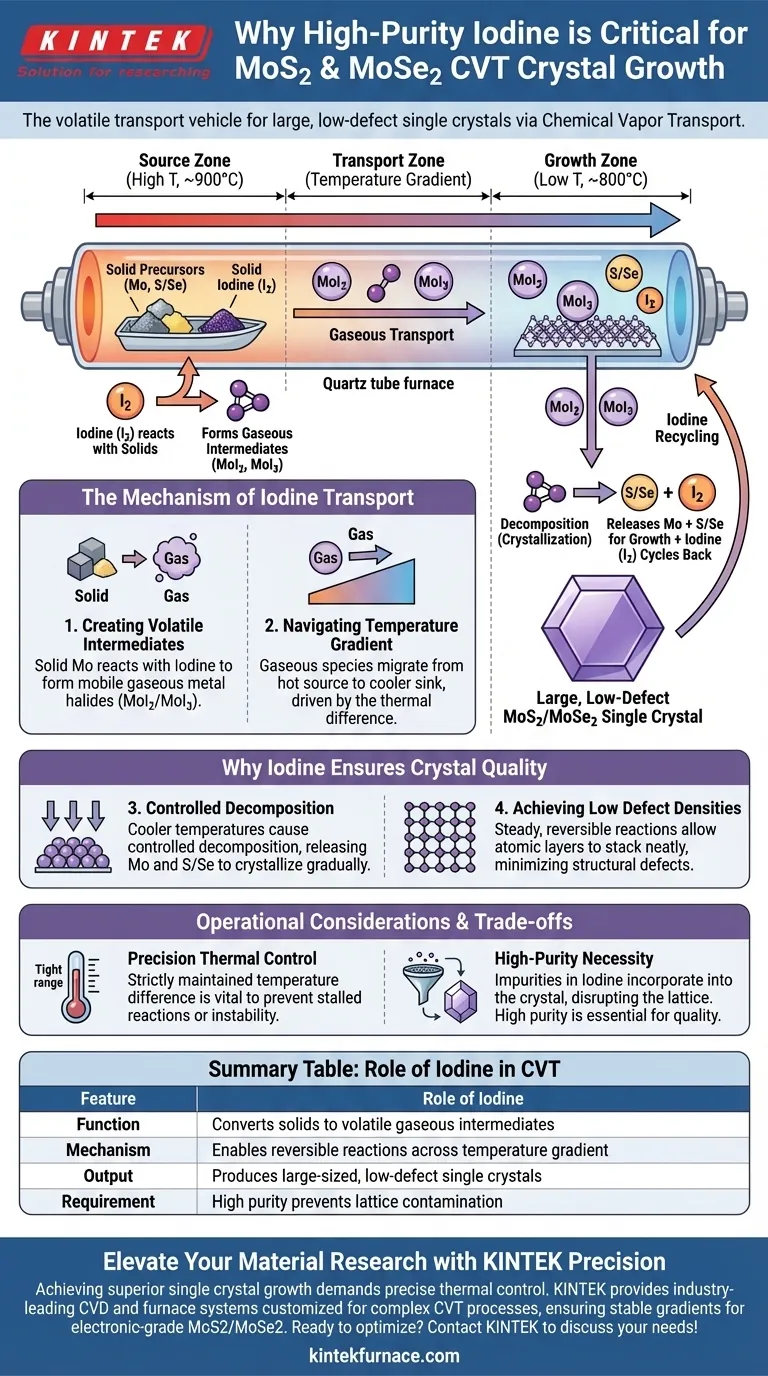
High-purity iodine serves as the critical volatile transport vehicle in the Chemical Vapor Transport (CVT) method. It functions by chemically reacting with solid Molybdenum and Sulfur (or Selenium) precursors to convert them into gaseous intermediates. This phase change allows the materials to migrate efficiently across the reactor to crystallize as Molybdenum Disulfide (MoS2) or Molybdenum Diselenide (MoSe2).
The core value of iodine lies in its ability to drive reversible chemical reactions. It bonds with solids to transport them as gases across a temperature gradient, then releases them to form large, high-quality single crystals with low defect densities.

The Mechanism of Iodine Transport
Creating Volatile Intermediates
Solid precursors, such as molybdenum and sulfur, are inherently stationary and cannot easily migrate to a growth zone on their own.
Iodine solves this by reacting with the solid molybdenum to form gaseous metal halide intermediates. Specifically, it facilitates the formation of species such as MoI2 and MoI3. These gaseous compounds are mobile and capable of traversing the reactor space.
Navigating the Temperature Gradient
The CVT process relies heavily on a controlled temperature difference within the reactor.
Once the iodine has converted the solids into gas-phase species at the "source" end, these gases migrate toward the cooler "sink" end of the tube. This movement is the fundamental "transport" mechanism that defines the CVT technique.
Why Iodine Ensures Crystal Quality
Controlled Decomposition
Upon reaching the cooler end of the reactor, the chemical environment changes due to the temperature drop.
Here, the gaseous metal halides (MoI2/MoI3) decompose. This decomposition releases the molybdenum and sulfur/selenium to react and crystallize, while the iodine is released back into the system to repeat the cycle.
Achieving Low Defect Densities
The use of iodine creates a highly stable growth environment.
Because the transport occurs via a steady, reversible reaction, the crystallization process happens gradually and strictly. This controlled pace allows the atomic layers of MoS2 or MoSe2 to stack neatly, resulting in large-sized single crystals that possess very few structural defects.
Operational Considerations and Trade-offs
The Necessity of Precision
While iodine is an effective transport agent, the process requires strict control over the thermal gradient.
If the temperature difference between the source and the sink is not maintained precisely, the reversible reactions may stall, or the transport rate may become unstable.
Purity Constraints
The reference emphasizes the use of high-purity iodine for a specific reason.
Any impurities present in the transport agent can become incorporated into the final crystal lattice. To achieve the low defect densities mentioned, the iodine source itself must be free of contaminants that could disrupt the crystal structure.
Making the Right Choice for Your Goal
To maximize the effectiveness of iodine in your CVT process, consider your specific end-goals:
- If your primary focus is Crystal Size: Ensure a stable and distinct temperature gradient to allow the iodine intermediates to transport material continuously without saturation.
- If your primary focus is Electronic Grade Quality: Verify the purity of your initial iodine source, as this directly correlates to the defect density of the final MoS2 or MoSe2 crystal.
Iodine is not just a carrier; it is the chemical regulator that defines the pace and quality of your crystal growth.
Summary Table:
| Feature | Role of Iodine in CVT |
|---|---|
| Function | Converts solid precursors into volatile gaseous intermediates (MoI2, MoI3) |
| Mechanism | Enables reversible chemical reactions across a temperature gradient |
| Output | Produces large-sized MoS2/MoSe2 single crystals with low defect density |
| Requirement | High purity is essential to prevent lattice contamination and structural defects |
Elevate Your Material Research with KINTEK Precision
Achieving superior single crystal growth requires more than just high-purity iodine; it demands precise thermal control. KINTEK provides industry-leading CVD and high-temperature furnace systems tailored for complex Chemical Vapor Transport (CVT) processes.
Backed by expert R&D and manufacturing, our customizable Tube, Vacuum, and CVD systems ensure the stable temperature gradients necessary for growing electronic-grade MoS2 and MoSe2.
Ready to optimize your lab's crystallization efficiency? Contact KINTEK today to discuss your custom furnace needs!
Visual Guide
References
- Bhupendra Mor, Kirti Korot. Comparative optical response and structural assessment of MoS₂ and MoSe₂ single crystals grown via iodine-assisted chemical vapor transport. DOI: 10.33545/26647575.2025.v7.i2a.168
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Cylindrical Resonator MPCVD Machine System for Lab Diamond Growth
- Magnesium Extraction and Purification Condensing Tube Furnace
- 915MHz MPCVD Diamond Machine Microwave Plasma Chemical Vapor Deposition System Reactor
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Multi Zone Laboratory Quartz Tube Furnace Tubular Furnace
People Also Ask
- Why does vacuum quality impact carbon chain yield? Essential Standards for High-Yield Synthesis
- What is the role of high-purity argon gas in ultrafine magnesium powder production? Control Particle Size & Purity
- How does a precision drying oven influence ZnO gel drying? Achieve Perfect Microporous Structures
- Why are different cooling methods compared for GFRP post-fire performance? Evaluate Thermal Shock & Safety Risks
- What technical advantages do high-temperature furnace systems provide for robust flexible interconnects? Enhance Durability
- What is the primary purpose of using a vacuum drying oven at 100°C? Optimize Aluminum Foil Coating Performance
- Why is a stainless steel high-pressure autoclave essential for starch hydrogenation? Unlock Peak Reaction Efficiency
- Why must ultra-high purity argon be continuously supplied for Aluminum-Silicon alloys? Ensure Viscosity Data Accuracy



















