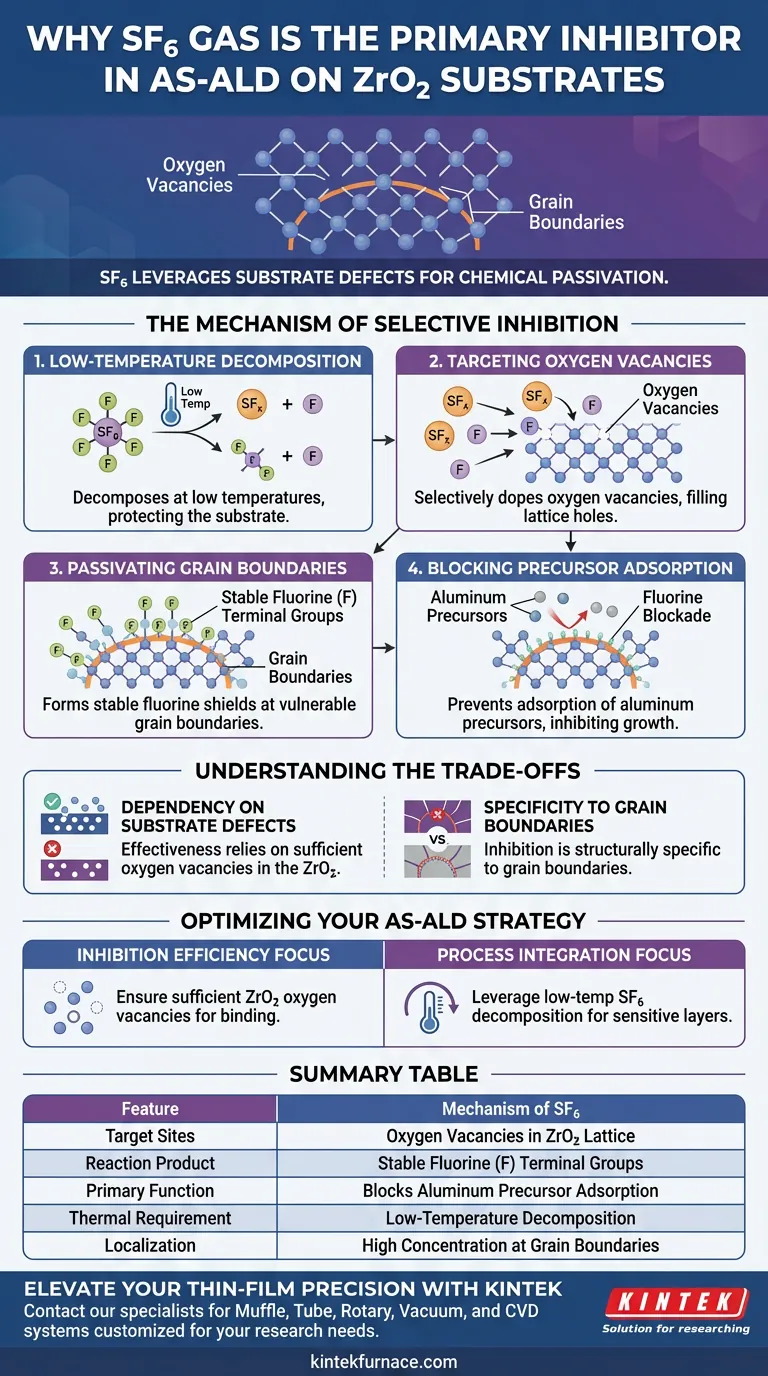
SF6 gas is chosen as the primary inhibitor due to its unique ability to leverage substrate defects for chemical passivation. It functions by decomposing at relatively low temperatures to selectively target oxygen vacancies within the Zirconia (ZrO2) lattice. This reaction creates stable fluorine terminal groups specifically at grain boundaries, which physically and chemically block the adsorption of aluminum precursors during subsequent deposition steps.
The power of SF6 lies in its precision: it does not merely coat the surface but actively modifies the substrate's defect sites. By converting oxygen vacancies into stable fluorine shields, it prevents unwanted material growth at the most vulnerable points—the grain boundaries.

The Mechanism of Selective Inhibition
Low-Temperature Decomposition
Unlike many passivation agents that require high thermal budgets, SF6 decomposes at relatively low temperatures. This characteristic is critical for maintaining the integrity of the underlying device structure during the AS-ALD process. It allows the inhibitor to activate and react without subjecting the substrate to excessive heat that could cause diffusion or damage.
Targeting Oxygen Vacancies
The efficacy of SF6 is driven by its interaction with specific defects in the Zirconia substrate. SF6 selectively dopes oxygen vacancies, effectively filling the "holes" in the crystal lattice. Rather than interacting uniformly across the entire material, the gas seeks out these specific chemical instabilities.
Passivating Grain Boundaries
The reaction at the vacancy sites results in the formation of stable fluorine (F) terminal groups. These groups are not randomly distributed; they form specifically at the grain boundaries of the ZrO2. This modifies the surface chemistry at the exact locations where unwanted nucleation typically begins.
Blocking Precursor Adsorption
Once established, these fluorine groups act as a chemical blockade. They prevent the adsorption of aluminum precursors, ensuring that the atomic layer deposition process is inhibited in the treated areas. This converts the grain boundaries from active nucleation sites into passive, non-reactive zones.
Understanding the Trade-offs
Dependency on Substrate Defects
Because the inhibition mechanism relies on doping oxygen vacancies, the process is highly dependent on the quality of the Zirconia substrate. A substrate with insufficient vacancy defects may not react as effectively with the SF6, potentially leading to incomplete inhibition.
Specificity to Grain Boundaries
The formation of fluorine groups is localized to grain boundaries. While this is effective for blocking diffusion paths, it implies that the inhibition is structurally specific. Areas away from grain boundaries or lacking defects may not receive the same level of passivation.
Optimizing Your AS-ALD Strategy
To effectively utilize SF6 for area-selective deposition, consider the state of your substrate and your thermal constraints.
- If your primary focus is Inhibition Efficiency: Ensure your ZrO2 substrate contains sufficient oxygen vacancies, as these are the necessary binding sites for the fluorine inhibitor.
- If your primary focus is Process Integration: Leverage the low-temperature decomposition of SF6 to passivate surfaces without exceeding the thermal budget of sensitive underlying layers.
By utilizing SF6, you convert the natural defects of Zirconia into a precise chemical mask, enabling high-fidelity selectivity where it matters most.
Summary Table:
| Feature | Mechanism of SF6 in AS-ALD |
|---|---|
| Target Sites | Oxygen Vacancies in ZrO2 Lattice |
| Reaction Product | Stable Fluorine (F) Terminal Groups |
| Primary Function | Blocks adsorption of aluminum precursors |
| Thermal Requirement | Low-temperature decomposition |
| Localization | High concentration at Grain Boundaries |
Elevate Your Thin-Film Precision with KINTEK
Are you looking to optimize your Area-Selective Atomic Layer Deposition or high-temperature material processing? At KINTEK, we understand that precision starts with the right environment. Backed by expert R&D and world-class manufacturing, we provide high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems—all fully customizable to meet the rigorous demands of advanced semiconductor and materials research.
Transform your laboratory's capabilities today. Contact our specialists now to discuss how our specialized thermal solutions can support your unique AS-ALD and substrate passivation needs.
Visual Guide
References
- Moo‐Yong Rhee, Il‐Kwon Oh. Area‐Selective Atomic Layer Deposition on Homogeneous Substrate for Next‐Generation Electronic Devices. DOI: 10.1002/advs.202414483
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- 2200 ℃ Graphite Vacuum Heat Treat Furnace
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- Laboratory Quartz Tube Furnace RTP Heating Tubular Furnace
- Multi Zone Laboratory Quartz Tube Furnace Tubular Furnace
People Also Ask
- Why is a stainless steel autoclave with a Teflon liner necessary for BiVO4? Ensure Purity & High Performance
- What role does an industrial-grade POCl3 diffusion furnace system play in DOSS? Master Quantitative Phosphorus Control
- What causes the increase in specific gravity of Moso Bamboo? Master Cellular Densification in Heat Treatment
- What is the objective of setting temperature gradients of 40 °C, 50 °C, and 60 °C? Optimize Yogurt Drying Viability
- What are the core technical advantages of an industrial microwave sintering system? Gain Speed and Material Integrity
- Why is a constant temperature drying oven necessary for CN/BOC-X composites? Ensure High Photocatalytic Activity
- Why is a constant temperature drying oven set to 60°C for 24 hours? Optimizing Sr4Al6O12SO4 Powder Quality
- What is the primary purpose of an industrial blast drying oven for Si/HC-X? Optimize Biomass Material Pretreatment



















