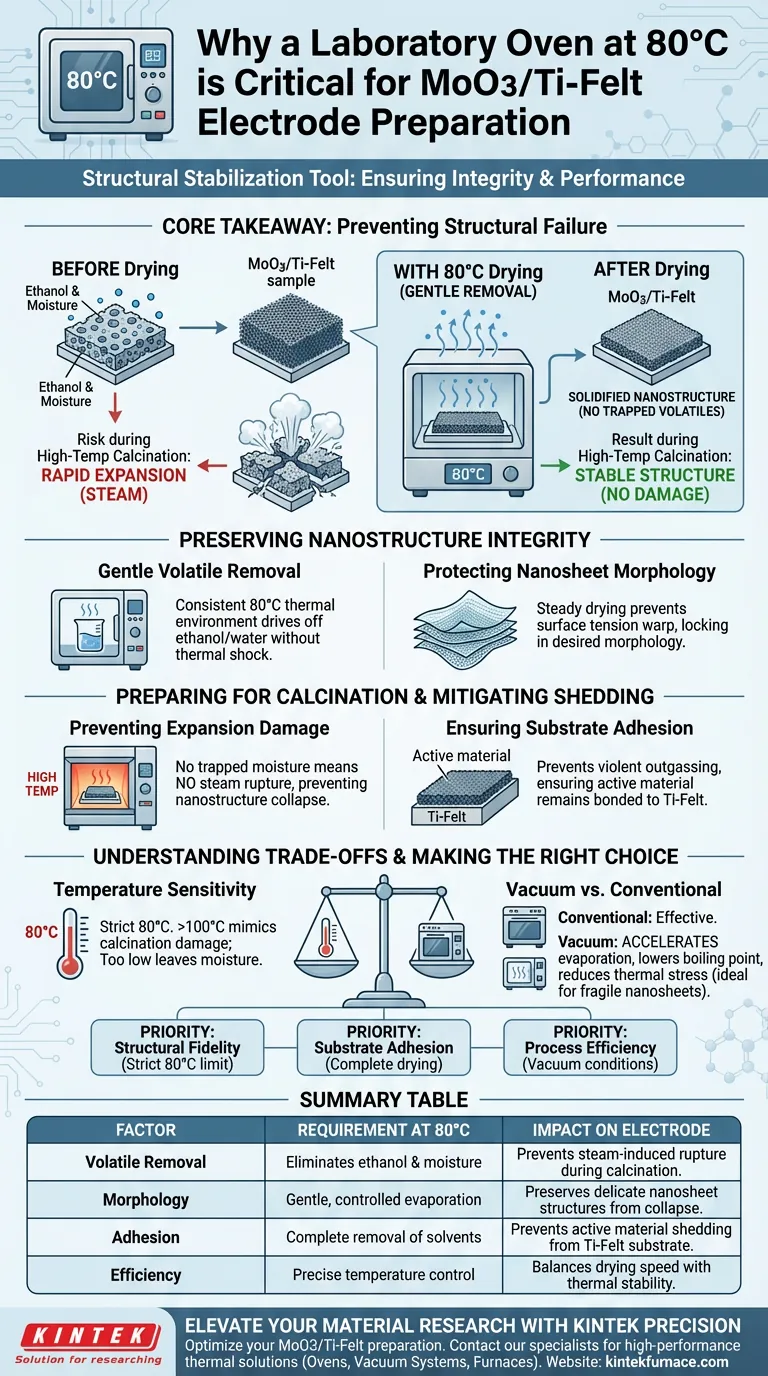
The laboratory oven serves as a critical structural stabilization tool during the preparation of MoO3/Ti-Felt electrodes. Specifically, drying samples at 80°C is required to completely remove residual absolute ethanol and moisture retained from the hydrothermal reaction. This controlled evaporation is the only way to solidify the initial nanostructure without causing physical damage to the delicate nanosheet morphology.
Core Takeaway The 80°C drying step is not simply about cleanliness; it is a vital preventative measure against structural failure. By gently removing volatiles now, you prevent the rapid expansion of trapped moisture during high-temperature calcination, which would otherwise cause the active material to shatter, collapse, or shed from the substrate.
Preserving Nanostructure Integrity
Gentle Removal of Volatiles
Following the hydrothermal reaction, your samples are saturated with absolute ethanol and water.
The laboratory oven provides a consistent thermal environment at 80°C. This specific temperature is sufficient to drive off these solvents effectively but remains low enough to avoid thermally shocking the material.
Protecting Nanosheet Morphology
The active material in these electrodes often consists of delicate nanosheets.
If solvents are not removed carefully, the surface tension forces during uncontrolled evaporation can warp or destroy these structures. The oven ensures a steady drying rate that "locks in" the desired morphology.
Preparing for High-Temperature Calcination
Preventing Rapid Expansion Damage
The most critical function of this drying step is preparing the sample for the subsequent calcination stage (often performed at much higher temperatures).
If moisture remains trapped inside the porous structure, the intense heat of calcination will turn that water into steam instantly. This rapid expansion creates internal pressure that can rupture the material, leading to a collapse of the nanostructure.
Mitigating Material Shedding
Adhesion to the Ti-Felt substrate is paramount for electrochemical performance.
By removing moisture prior to calcination, you prevent the violent outgassing that often causes the active material to physically detach or "shed" from the current collector. This ensures the active layer remains firmly bonded to the titanium felt.
Understanding the Trade-offs
Temperature Sensitivity
It is crucial to adhere strictly to the 80°C set point.
Drying at significantly higher temperatures (e.g., >100°C) initially can induce rapid solvent boiling, which mimics the damage caused by calcination. Conversely, temperatures that are too low may fail to remove strongly adsorbed moisture, leaving the sample vulnerable during the next processing step.
Vacuum vs. Conventional Drying
While a conventional oven works, using a vacuum oven at this stage offers distinct advantages.
Vacuum conditions lower the boiling point of solvents, accelerating evaporation without increasing thermal stress. This is particularly useful if your nanosheets are exceptionally fragile or if you wish to minimize the risk of thermal oxidation.
Making the Right Choice for Your Goal
When configuring your drying protocol for MoO3/Ti-Felt electrodes, consider your specific priorities:
- If your primary focus is Structural Fidelity: Maintain a strict 80°C limit to preserve nanosheet morphology and prevent pore collapse.
- If your primary focus is Substrate Adhesion: Ensure drying is complete (no residual moisture) to prevent delamination caused by steam expansion during calcination.
- If your primary focus is Process Efficiency: Utilize vacuum conditions to speed up solvent removal without raising the temperature, ensuring safety and speed.
Proper drying at 80°C is the unsung hero of electrode synthesis, transforming a fragile precursor into a robust, high-performance material.
Summary Table:
| Factor | Requirement at 80°C | Impact on Electrode |
|---|---|---|
| Volatile Removal | Eliminates ethanol & moisture | Prevents steam-induced rupture during calcination |
| Morphology | Gentle, controlled evaporation | Preserves delicate nanosheet structures from collapse |
| Adhesion | Complete removal of solvents | Prevents active material shedding from Ti-Felt substrate |
| Efficiency | Precise temperature control | Balances drying speed with thermal stability |
Elevate Your Material Research with KINTEK Precision
Don't let improper drying compromise your electrode's performance. Backed by expert R&D and manufacturing, KINTEK offers high-performance laboratory ovens, vacuum systems, and customizable high-temp furnaces (Muffle, Tube, Rotary, CVD) designed to meet the rigorous demands of advanced material synthesis.
Ready to optimize your MoO3/Ti-Felt preparation? Contact our specialists today to find the perfect thermal solution for your lab's unique research needs.
Visual Guide
References
- Electrocatalytic Hydrogen Generation from Seawater at Neutral pH on a Corrosion-Resistant MoO<sub>3</sub>/Ti-Felt Electrode. DOI: 10.1021/acssuschemeng.5c02839
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1200℃ Muffle Oven Furnace for Laboratory
- Vacuum Hot Press Furnace Machine Heated Vacuum Press Tube Furnace
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
People Also Ask
- How does Faraday's Law of Induction work in induction heating? Achieve Precise, Non-Contact Thermal Processing
- What are the key advantages of using an annealing furnace? Enhance Material Quality and Manufacturing Efficiency
- How does a box heater work? A Guide to Efficient Whole-Room Heating
- What is the significance of using a hydrogen etching process in a reaction chamber? Mastering SiC Surface Preparation
- What role does precision analytical equipment play in petrochemical R&D? Engineering the Future of Efficient Refining
- What is the primary function of a laboratory electric drying oven in sample prep? Ensure Pure, Grinder-Ready Powders
- What is the purpose of silver paste coating for BCZT ceramics? Ensuring Precision in Electrical Performance Testing
- Why is a precision furnace required after TiO2-alpha-Ga2O3 synthesis? Master Phase Transformation & Interface Bonding



















