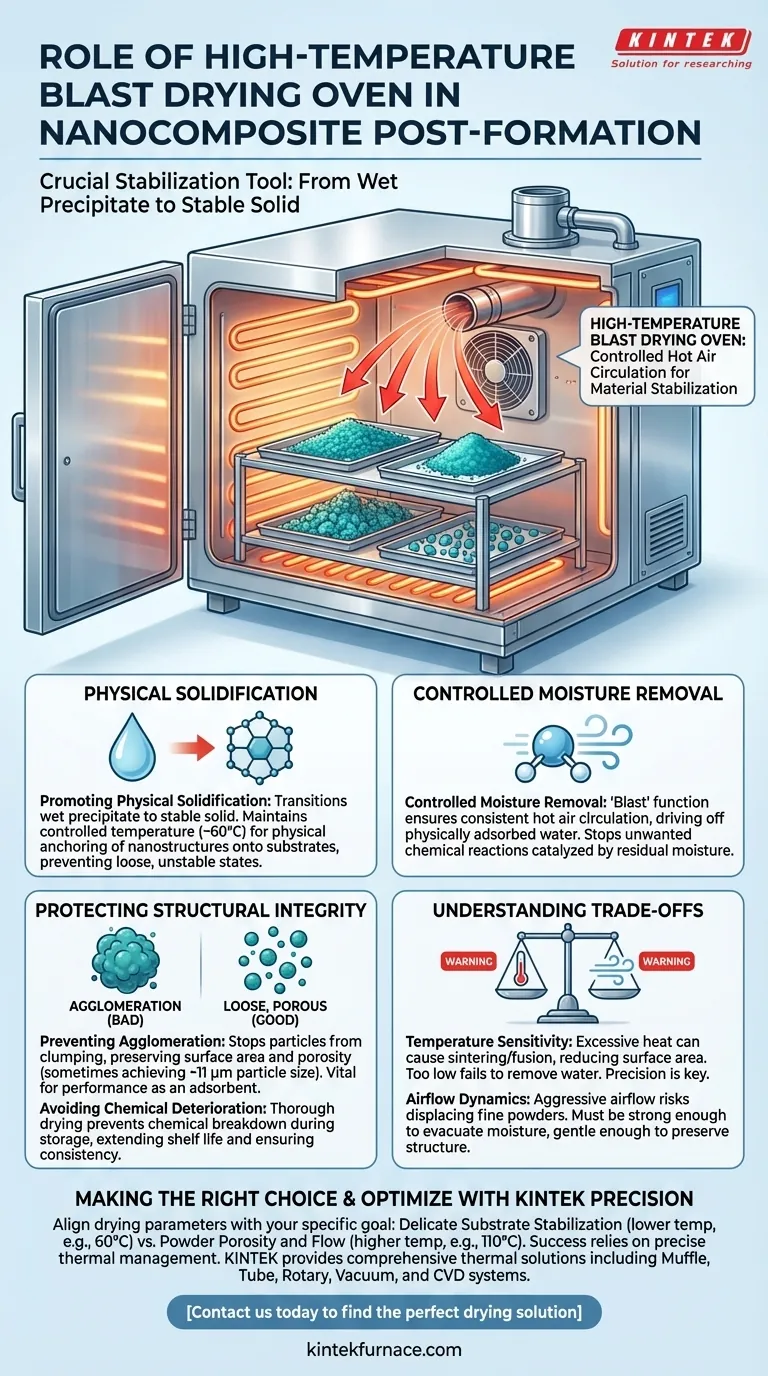
The high-temperature blast drying oven serves as a crucial stabilization tool in the post-formation of nanocomposites. It utilizes controlled hot air circulation to systematically remove residual moisture from composite precipitates, ensuring the physical solidification of nanostructures onto their substrates while preventing material degradation.
By effectively managing the thermal environment, this equipment bridges the gap between raw synthesis and a usable product. It secures the structural integrity of the material by eliminating the moisture that drives agglomeration and chemical instability.

Mechanisms of Material Stabilization
Promoting Physical Solidification
The primary function of the oven is to transition the material from a wet precipitate to a stable solid.
By maintaining a controlled temperature (often around 60°C for delicate precipitates), the oven facilitates the physical anchoring of nanostructures. This ensures they adhere properly to the substrate rather than remaining in a loose or unstable state.
Controlled Moisture Removal
Residual moisture is a significant threat to nanocomposite quality.
The "blast" function ensures consistent hot air circulation, which drives off physically adsorbed water. This dehydration is essential for stopping unwanted chemical reactions that water might catalyze after the initial formation.
Protecting Structural Integrity
Preventing Agglomeration
One of the most critical roles of the drying process is to stop particles from clumping together.
Without precise drying, nanoparticles tend to aggregate, destroying the desired surface area and porosity. Proper drying yields a loose, porous material—sometimes achieving specific particle sizes around 11 μm—which is vital for the material's performance as an adsorbent.
Avoiding Chemical Deterioration
Moisture trapped within a nanocomposite can lead to rapid degradation during storage.
By thoroughly drying the precipitates, the oven prevents chemical breakdown. This extends the shelf life of the material and ensures its properties remain consistent from the lab to the application site.
Understanding the Trade-offs
Temperature Sensitivity
While heat is necessary for drying, excessive temperatures can be detrimental.
If the temperature is set too high, it may cause the nanostructures to sinter or fuse, reducing their active surface area. Conversely, temperatures that are too low will fail to remove all adsorbed water, leading to instability.
Airflow Dynamics
The "blast" aspect refers to forced air circulation, which promotes uniformity but introduces physical force.
If the airflow is too aggressive, it risks displacing fine powders or creating inconsistencies in the drying bed. The circulation must be strong enough to evacuate moisture but gentle enough to preserve the physical structure of the precipitate.
Making the Right Choice for Your Goal
To maximize the effectiveness of your post-formation process, align your drying parameters with your specific material needs:
- If your primary focus is Delicate Substrate Stabilization: Utilize lower controlled temperatures (e.g., 60°C) to slowly solidify nanostructures without thermal shock.
- If your primary focus is Powder Porosity and Flow: Employ higher temperatures (e.g., 110°C) to ensure complete dehydration and prevent particle agglomeration.
Success in nanocomposite fabrication relies not just on synthesis, but on the precise thermal management that locks in your material's final properties.
Summary Table:
| Process Function | Impact on Nanocomposites | Key Mechanism |
|---|---|---|
| Physical Solidification | Anchors nanostructures to substrates | Controlled thermal anchoring at ~60°C |
| Moisture Removal | Prevents chemical degradation and instability | Forced air circulation (blast function) |
| Agglomeration Control | Maintains high surface area and porosity | Uniform drying to prevent particle clumping |
| Thermal Optimization | Prevents sintering or structural fusion | Precision temperature management |
Optimize Your Nanocomposite Synthesis with KINTEK Precision
Ensure the structural integrity and performance of your advanced materials with our high-performance thermal solutions. Backed by expert R&D and world-class manufacturing, KINTEK provides a comprehensive range of lab equipment—including Muffle, Tube, Rotary, Vacuum, and CVD systems—all customizable to meet your specific research or production needs.
Don't let improper drying compromise your lab's results. Contact us today to find the perfect drying solution for your unique application.
Visual Guide
References
- Büşra Şensoy Gün, Belgin Tunalı. Biofilm-inhibiting ZnO@Eggshell nanocomposites: green synthesis, characterization, and biomedical potential. DOI: 10.1007/s10534-025-00711-8
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1200℃ Muffle Oven Furnace for Laboratory
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Molybdenum Disilicide MoSi2 Thermal Heating Elements for Electric Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
People Also Ask
- How does high-purity argon gas affect the production of ultrafine magnesium powder in evaporation-condensation methods? Master Particle Size Control
- What is the function of a fixed-bed catalytic reactor in ex situ CHP? Optimize Your Bio-oil Quality Today
- What are the advantages of using a microwave reaction system? Rapid & Uniform Synthesis of Doped Hydroxyapatite
- What are the primary applications of a constant temperature drying oven? Master Biochar Selenium-Modification
- Why is thermogravimetric analysis (TGA) necessary for modified hard carbon? Optimize Stability & Composition
- Why is a high-precision programmed heating system used for catalyst stability? Ensure Precise Reactor Data Integrity
- Why do high-performance Bi-2223 superconducting materials require high-precision temperature control? | KINTEK Solution
- What is the specific function of a high-temperature laboratory furnace during the activation of kaolin-based catalysts?



















