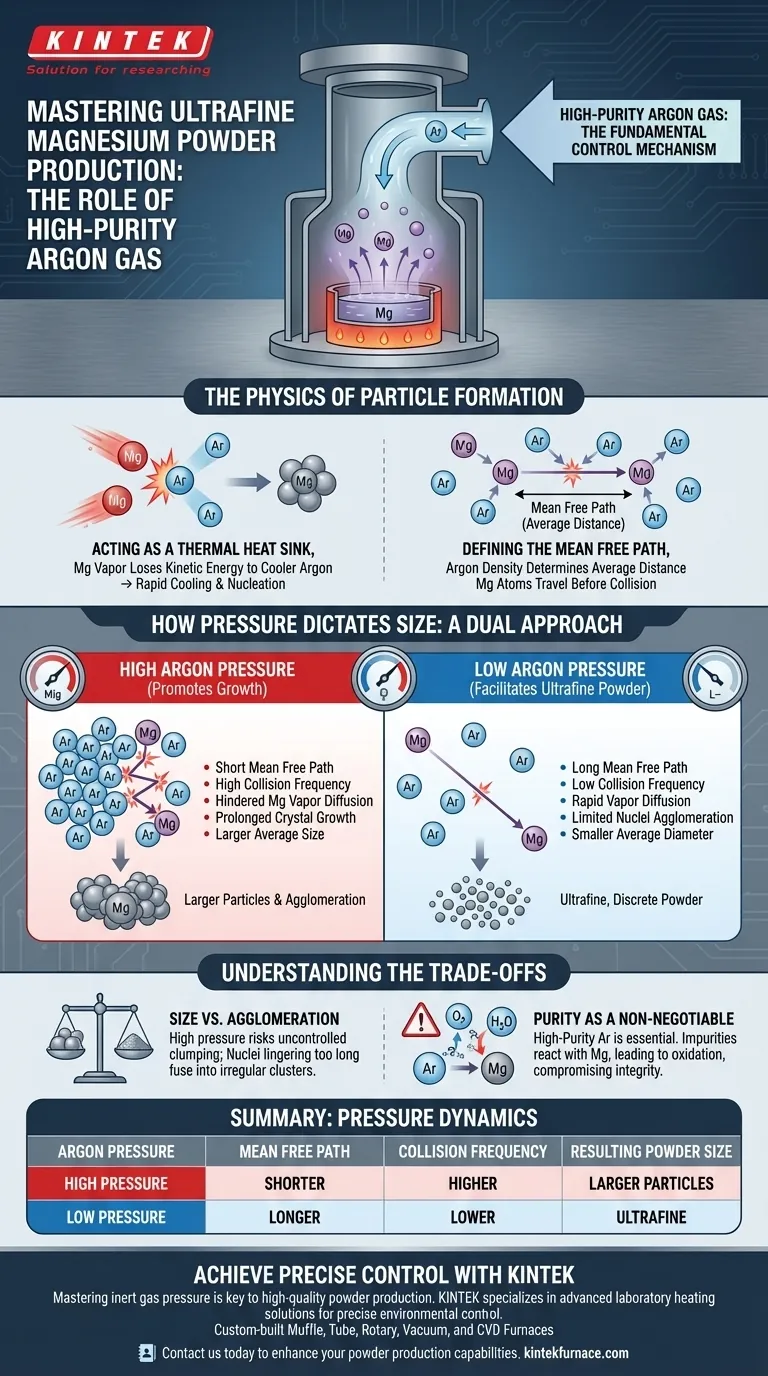
High-purity argon gas serves as the fundamental control mechanism for determining the size and quality of magnesium powder in evaporation-condensation processes. It acts as an inert "brake" and thermal sink for magnesium vapor atoms. By manipulating the pressure of this gas, you directly influence how magnesium atoms collide, cool, and eventually cluster into solid particles.
The central principle is that argon pressure dictates the "mean free path" of magnesium atoms. Controlling this variable allows you to precisely tune the balance between rapid nucleation (creating many small particles) and prolonged crystal growth (creating fewer, larger particles).

The Physics of Particle Formation
Acting as a Thermal Heat Sink
In the evaporation-condensation method, magnesium starts as a high-energy vapor. Before it can become a powder, it must lose kinetic energy.
High-purity argon provides the cool environment necessary for this phase change. As hot magnesium atoms collide with cooler argon atoms, they transfer energy, resulting in rapid cooling and the nucleation of solid crystals.
Defining the Mean Free Path
The critical variable in this process is the mean free path. This is the average distance a magnesium atom travels before colliding with an argon atom.
The density of the argon gas determines this distance. A shorter path means more frequent collisions, while a longer path allows magnesium atoms to travel further without interference.
How Pressure Dictates Size
High Pressure Promotes Growth
When you increase the pressure of the argon gas, you increase the number of argon atoms in the chamber. This drastically creates a shorter mean free path and increases the collision frequency.
High pressure hinders the diffusion of magnesium vapor. Because the vapor cannot disperse quickly, crystal nuclei remain in the growth zone longer. This allows them to grow larger through adsorption and agglomeration, resulting in a larger average particle size.
Low Pressure Facilitates Ultrafine Powder
Conversely, to achieve ultrafine powder, the process generally requires lower argon pressure. Lower pressure increases the mean free path, allowing vapor to diffuse more rapidly.
This rapid diffusion prevents the nuclei from colliding and clumping together (agglomeration) as frequently. The result is a finer, more discrete powder with a smaller average particle diameter.
Understanding the Trade-offs
Size vs. Agglomeration
While high pressure is effective for creating larger, distinct crystals, it increases the risk of uncontrolled agglomeration. If the nuclei linger too long in a dense gas cloud, they may fuse into irregular clusters rather than spherical particles.
Purity as a Non-Negotiable
The reference specifically highlights high-purity argon. This is not merely a preference; it is a chemical necessity. Magnesium is highly reactive.
Any impurities in the carrier gas (such as oxygen or moisture) will react with the magnesium vapor before it condenses. This compromises the integrity of the powder, leading to oxidation rather than pure metallic magnesium.
Making the Right Choice for Your Goal
Adjusting argon pressure is the most effective way to shift your production outcome.
- If your primary focus is Ultrafine Powder: Maintain lower argon pressures to increase the mean free path and limit the time available for crystal growth and agglomeration.
- If your primary focus is Larger Particle Size: Increase argon pressure to raise collision frequency, hindering diffusion and encouraging nuclei to grow into larger crystals.
Mastering the pressure dynamics of argon allows you to move from random results to a predictable, tunable manufacturing process.
Summary Table:
| Argon Pressure | Mean Free Path | Collision Frequency | Resulting Powder Size | Key Characteristic |
|---|---|---|---|---|
| Low Pressure | Longer | Lower | Ultrafine | Rapid diffusion, limited agglomeration |
| High Pressure | Shorter | Higher | Larger Particles | Slower diffusion, promotes crystal growth |
Ready to Achieve Precise Control Over Your Powder Production?
Mastering the dynamics of inert gas pressure is key to producing high-quality, consistent metal powders. At KINTEK, we specialize in advanced laboratory heating solutions that provide the precise environmental control your R&D and manufacturing processes demand.
Our custom-built Muffle, Tube, Rotary, Vacuum, and CVD furnaces are engineered for exact temperature and atmosphere management, crucial for evaporation-condensation and other sensitive powder synthesis methods. Whether your goal is ultrafine magnesium powder or larger, distinct crystals, our systems can be tailored to your unique requirements.
Contact us today to discuss how our expertise and customizable furnace systems can enhance your powder production capabilities and deliver the predictable, high-quality results you need.
Visual Guide
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Split Chamber CVD Tube Furnace with Vacuum Station CVD Machine
People Also Ask
- How is a high-temperature tube furnace utilized in the synthesis of MoO2/MWCNTs nanocomposites? Precision Guide
- What are the key operational considerations when using a lab tube furnace? Master Temperature, Atmosphere & Safety
- What safety and reliability features are incorporated into a vertical tube furnace? Ensuring Safe, Consistent High-Temp Processing
- What safety measures are essential when operating a lab tube furnace? A Guide to Preventing Accidents
- What is an example of a material prepared using a tube furnace? Master Precise Material Synthesis



















