Thermogravimetric analysis (TGA) is the definitive method for determining the thermal stability and precise chemical composition of modified hard carbon. By continuously monitoring mass changes as the material is heated, TGA provides critical data on actual sulfur content, distinguishing between loose surface adsorption and robust chemical bonding.
TGA is essential for optimizing the synthesis of sulfur-modified hard carbon. It not only quantifies the actual sulfur loading but also identifies the specific temperature limits where chemical bonds break, enabling the selection of the ideal carbonization temperature to ensure material stability.

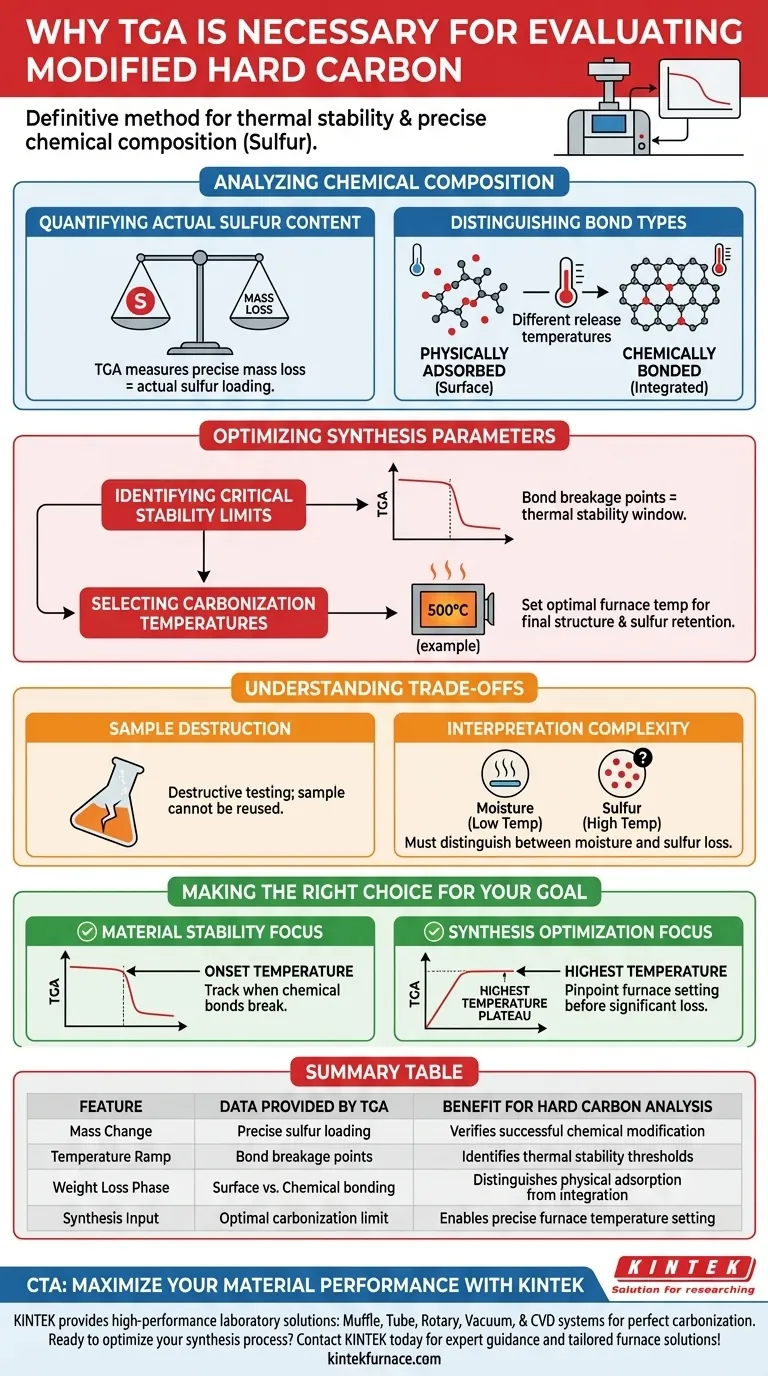
Analyzing Chemical Composition
Quantifying Actual Sulfur Content
To evaluate modified hard carbon effectively, you must know exactly how much sulfur has been successfully incorporated into the material.
TGA measures the precise mass loss during heating, which correlates directly to the actual sulfur content. This verifies whether the modification process achieved the desired chemical loading.
Distinguishing Bond Types
Not all sulfur within the hard carbon matrix behaves the same way.
TGA allows you to differentiate between physically adsorbed sulfur (trapped on the surface) and chemically bonded sulfur (integrated into the carbon structure). This distinction is made by observing the different temperatures at which these forms of sulfur are released.
Optimizing Synthesis Parameters
Identifying Critical Stability Limits
To prevent material degradation, you must identify the thermal limits of your modified carbon.
TGA identifies the critical temperatures responsible for carbon-sulfur bond breakage and subsequent sulfur loss. This data maps the thermal stability window of the material.
Selecting Carbonization Temperatures
The ultimate goal of TGA in this context is to inform the manufacturing process.
By analyzing the stability data, researchers can select the optimal carbonization temperature (such as 500 °C). This ensures the synthesis temperature is high enough to finalize the structure but low enough to prevent the loss of active sulfur components.
Understanding the Trade-offs
Sample Destruction
It is important to note that TGA is a destructive testing method. The heating process burns off the sulfur and modifies the carbon, meaning the specific sample used for analysis cannot be recovered or reused.
Interpretation Complexity
While TGA provides precise mass loss data, interpreting the cause of the loss requires context.
Users must be careful to distinguish between mass loss caused by moisture evaporation at lower temperatures and the loss of the target modifier (sulfur) at higher temperatures. Misinterpreting these signals can lead to incorrect calculations of sulfur content.
Making the Right Choice for Your Goal
To get the most out of your TGA data when evaluating modified hard carbon, align your analysis with your specific objective:
- If your primary focus is Material Stability: Concentrate on the onset temperature of the second major weight loss event, as this indicates where chemical bonds begin to break.
- If your primary focus is Synthesis Optimization: Use the TGA curve to pinpoint the highest temperature plateau before significant sulfur loss occurs to set your carbonization furnace (e.g., confirming the 500 °C target).
Use TGA not just to measure what you have, but to define how you make it.
Summary Table:
| Feature | Data Provided by TGA | Benefit for Hard Carbon Analysis |
|---|---|---|
| Mass Change | Precise sulfur loading | Verifies successful chemical modification |
| Temperature Ramp | Bond breakage points | Identifies thermal stability thresholds |
| Weight Loss Phase | Surface vs. Chemical bonding | Distinguishes physical adsorption from integration |
| Synthesis Input | Optimal carbonization limit | Enables precise furnace temperature setting (e.g. 500°C) |
Maximize Your Material Performance with KINTEK
Precise thermogravimetric analysis demands reliable heating environments. KINTEK provides high-performance laboratory solutions—including Muffle, Tube, Rotary, Vacuum, and CVD systems—engineered to help you achieve perfect carbonization and material stability. Backed by expert R&D and manufacturing, our equipment is fully customizable to meet the unique requirements of your hard carbon research.
Ready to optimize your synthesis process? Contact KINTEK today for expert guidance and tailored furnace solutions!
Visual Guide
References
- Yuanfeng Liu, Yong Wang. Shredded-Coconut-Derived Sulfur-Doped Hard Carbon via Hydrothermal Processing for High-Performance Sodium Ion Anodes. DOI: 10.3390/nano15100734
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
- 2200 ℃ Graphite Vacuum Heat Treat Furnace
- Vacuum Heat Treat Sintering and Brazing Furnace
- Molybdenum Vacuum Heat Treat Furnace
People Also Ask
- What is the technical objective of performing thermal oxidation at 625 °C? Mastering SiOx Tunnel Oxide Precision
- What is the function of planetary ball mills or industrial mixing granulators prior to RHF? Optimize FMDS Reactivity
- Why is a rapid cooling process necessary for BZSM nanophosphors? Secrets of Metastable Phase Retention
- How does a high-temperature sintering furnace influence ZnO nanotube sensors? Unlock Peak Sensitivity and Stability
- What role does an RTA system play in Zirconia preparation? Master Phase Transformation for Advanced Deposition
- What is the function of an inert gas supply system in black liquor pyrolysis? Achieve Precise Atmospheric Control
- How do heating rate and holding temperature influence Zr2.5Nb nitride growth? Optimize Your ZrN Layer Thickness
- What are the advantages of using a high-pressure oxygen annealing furnace for La1-xSrxMnO3 thin films?



















