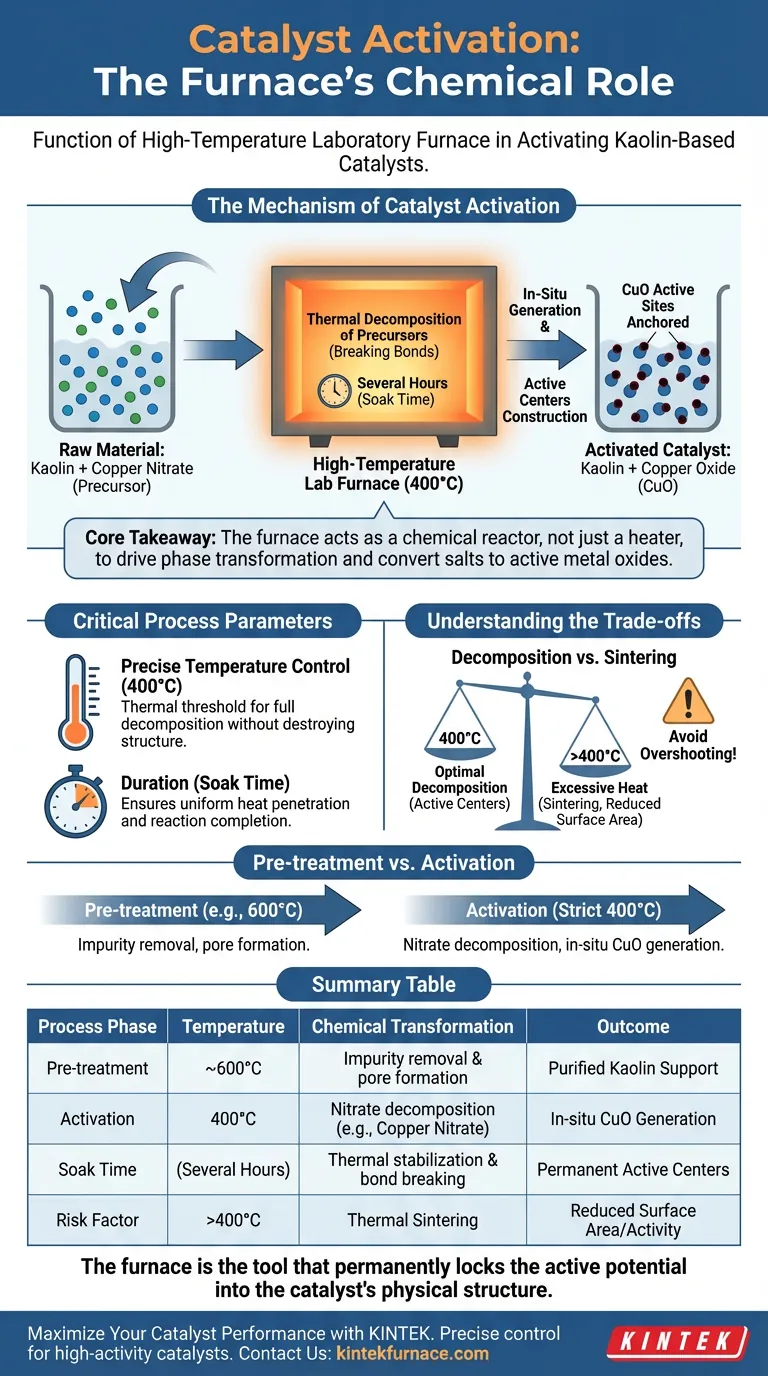
The specific function of a high-temperature laboratory furnace during the activation of kaolin-based catalysts is to facilitate the chemical decomposition of metal precursors into active catalytic agents.
Specifically, for kaolin loaded with copper nitrate, the furnace maintains a constant temperature of 400°C for several hours. This thermal treatment decomposes the copper nitrate, resulting in the in-situ generation of copper oxide (CuO). This conversion is the defining step that constructs the active centers on the support structure, effectively turning an inert mixture into a functional catalyst.
Core Takeaway The furnace acts not merely as a heating device, but as a chemical reactor that drives the phase transformation of catalyst precursors. Its primary role is to convert metal salts (like copper nitrate) into active metal oxides (like CuO) through precise calcination, anchoring them to the kaolin support to create stable active sites.

The Mechanism of Catalyst Activation
The activation process is a chemical transformation driven by heat. Understanding what happens inside the furnace explains why this equipment is the linchpin of catalyst preparation.
Thermal Decomposition of Precursors
The raw material entering the furnace is kaolin clay impregnated with a metal salt, typically copper nitrate. In its raw form, this salt is catalytically inactive. The furnace provides the energy required to break the chemical bonds of the nitrate.
In-Situ Generation of Active Phases
At a sustained temperature of 400°C, the nitrate compounds break down. This process leaves behind copper oxide (CuO) directly on the surface of the kaolin. Because this happens "in-situ" (in place), the oxide forms intimately with the support structure, rather than being mechanically mixed in later.
Construction of Active Centers
The ultimate goal of this heating cycle is the creation of active centers. These are the specific atomic sites where future chemical reactions will occur. Without this thermal treatment, the material would simply be clay covered in salt; the furnace transforms it into a structured material capable of accelerating chemical reactions.
Critical Process Parameters
For the activation to be successful, the furnace must provide more than just high heat; it must provide a controlled environment.
Precise Temperature Control
The target temperature of 400°C is not arbitrary. It is the specific thermal threshold required to fully decompose the copper nitrate without destroying the underlying kaolin structure. The furnace must maintain this temperature accurately to ensure the chemical conversion is uniform across the entire batch.
Duration and Thermal Stability
The process requires the temperature to be held constant for several hours. This "soak time" ensures that heat penetrates the core of the material and that the decomposition reaction reaches completion. If the furnace temperature fluctuates, the resulting catalyst may have uneven activity or incomplete active sites.
Understanding the Trade-offs
While the furnace is essential for activation, improper use or equipment selection can degrade the catalyst's performance.
Decomposition vs. Sintering
There is a delicate balance in thermal treatment. You must apply enough heat to decompose the precursor (400°C), but excessive heat or overshooting the temperature can lead to sintering. Sintering causes the active metal particles to clump together, drastically reducing the surface area and the catalyst's effectiveness.
Pre-treatment vs. Activation
It is important to distinguish between pre-treating the raw support and activating the final catalyst. While raw natural kaolin might be calcined at higher temperatures (e.g., 600°C) to remove impurities and increase porosity, the activation of the copper-loaded catalyst occurs at a lower, stricter temperature (400°C). Confusing these two setpoints can destroy the active copper phase.
Making the Right Choice for Your Goal
To maximize the performance of your kaolin-based catalysts, apply these principles to your thermal processing strategy:
- If your primary focus is Chemical Activity: Ensure your furnace can hold exactly 400°C without fluctuation to guarantee the complete conversion of copper nitrate to copper oxide (CuO).
- If your primary focus is Batch Consistency: Verify that your furnace has excellent thermal field stability so that every gram of material receives the same thermal history, preventing "dead spots" in the catalyst bed.
- If your primary focus is Structural Integrity: Avoid exceeding the necessary activation temperature; higher heat does not mean better activation and often leads to the collapse of the catalyst's pore structure.
The furnace is the tool that permanently locks the active potential into the catalyst's physical structure.
Summary Table:
| Process Phase | Temperature | Chemical Transformation | Outcome |
|---|---|---|---|
| Pre-treatment | ~600°C | Impurity removal & pore formation | Purified Kaolin Support |
| Activation | 400°C | Nitrate decomposition (e.g., Copper Nitrate) | In-situ CuO Generation |
| Soak Time | Several Hours | Thermal stabilization & bond breaking | Permanent Active Centers |
| Risk Factor | >400°C | Thermal Sintering | Reduced Surface Area/Activity |
Maximize Your Catalyst Performance with KINTEK
Precise temperature control is the difference between a high-activity catalyst and a failed batch. At KINTEK, we understand that thermal stability is critical for the delicate in-situ generation of active centers on kaolin supports.
Why Choose KINTEK?
- Expert R&D & Manufacturing: Our systems are designed to prevent temperature overshooting and sintering.
- Versatile Solutions: Whether you need Muffle, Tube, Rotary, Vacuum, or CVD systems, we provide the tools required for sophisticated calcination.
- Customizable for Your Needs: We tailor our lab high-temp furnaces to meet your specific thermal threshold and soak time requirements.
Ensure your catalyst precursors transform into stable, high-performance active sites with our industry-leading technology.
Contact KINTEK Today to Enhance Your Lab's Efficiency
Visual Guide
References
- Mohammed Alhassan, U. Shamsideen. PRODUCTION OF BIOLUBRICANT BLEND FROM JATROPHA CURCAS OIL. DOI: 10.33003/fjs-2023-0706-2168
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ Muffle Oven Furnace for Laboratory
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
People Also Ask
- How does nano-MgO particle size influence sulfur doping in activated carbon? Optimize Doping for High-Performance Lab Materials
- How does the speed-controlled motor in a high-pressure autoclave influence the yield of glucose from starch?
- How does uniform heating benefit furnace applications? Achieve Superior Quality and Efficiency
- What preparatory questions should be addressed before converting to electrically heated processes? Ensure a Smooth Transition to Electric Heating
- How does a precision pressure-controlled oxidation device increase carbon chain yield? Optimize Your Annealing Process
- Why is an equivalent diffusion combustion heat source term integrated into the furnace temperature field simulation?
- What is the specific function of laboratory electric heating devices in solid-state hydrogen storage? Optimize Thermal Management
- How is the success of stress-relief heat treatment in AlSi10Mg verified? Ensure Part Integrity with XRD



















