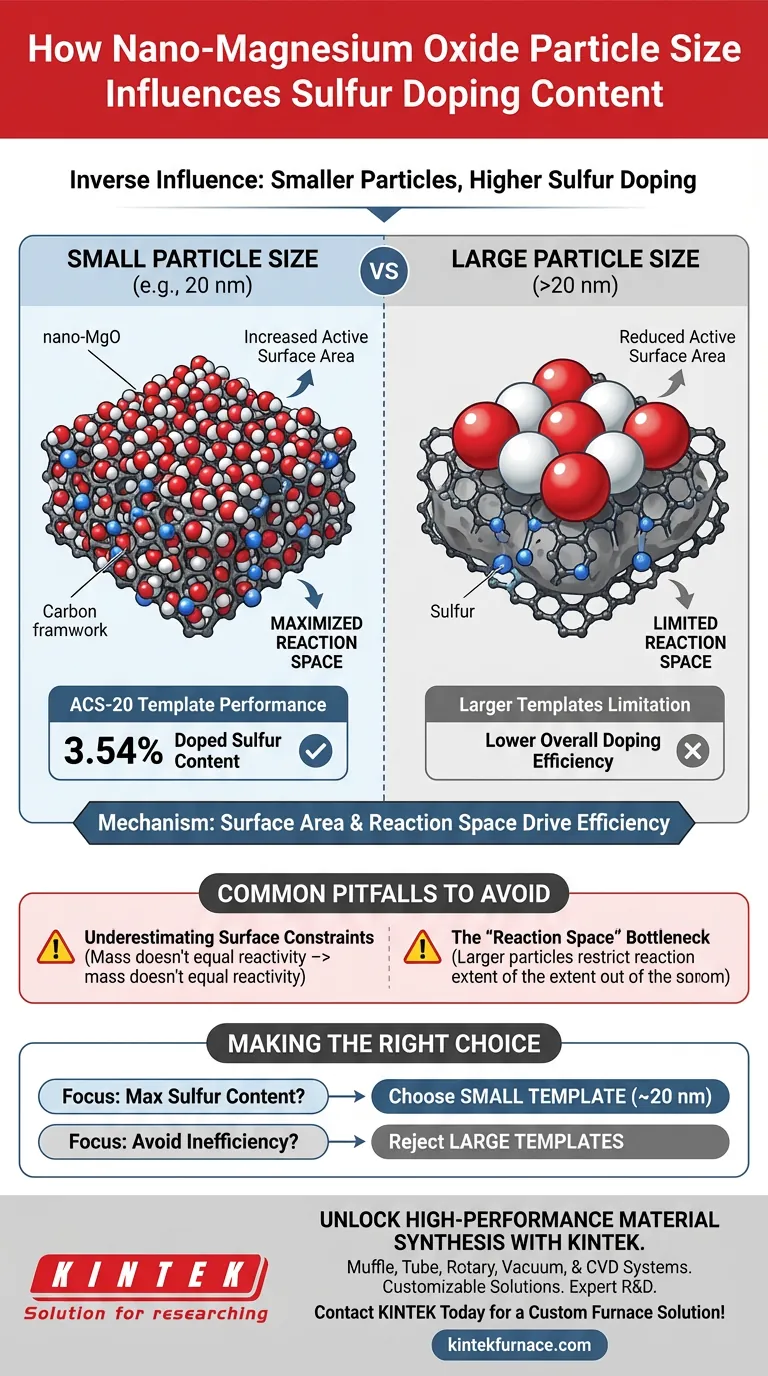
The particle size of nano-magnesium oxide exerts a direct, inverse influence on sulfur doping content. Specifically, utilizing smaller particle sizes, such as 20 nm, significantly increases the percentage of sulfur incorporated into the activated carbon. This is primarily driven by the dramatic increase in available active surface area provided by smaller particles.
The core principle is that smaller template particles maximize the "reaction space" per unit of mass. This increased surface exposure facilitates a more complete reaction between the carbon framework and the sulfur source, resulting in superior doping efficiency.

The Mechanism Behind Doping Efficiency
The Role of Active Surface Area
The fundamental driver of this process is the active surface area. Smaller nano-magnesium oxide particles provide a much larger surface area for the same mass compared to larger particles.
Facilitating the Reaction
This expanded surface area allows for greater interaction between materials. It ensures a more comprehensive reaction between the carbon framework and the sulfur source.
Creating Reaction Space
Smaller particles effectively increase the available reaction space. This physical characteristic removes bottlenecks that would otherwise prevent sulfur from integrating into the carbon structure.
Evidence of Impact
Performance of 20 nm Templates (ACS-20)
Empirical studies demonstrate clear advantages when using smaller templates. Specifically, sulfur-doped porous carbon prepared with a 20 nm template (ACS-20) achieves a high doped sulfur content of approximately 3.54%.
The Limitation of Larger Templates
Conversely, larger templates result in reduced doping content. The larger particle size inherently limits the active reaction space, leading to lower overall doping efficiency.
Common Pitfalls to Avoid
Underestimating Surface Constraints
A common error in synthesis is assuming that mass equals reactivity. Even if the mass of the magnesium oxide is constant, increasing the particle size reduces the functional surface area available for the reaction.
The "Reaction Space" Bottleneck
Using larger particles creates a physical constraint. This limits the extent of the reaction between the carbon and sulfur, making it chemically impossible to achieve the high doping levels seen with 20 nm particles.
Making the Right Choice for Your Goal
To optimize the synthesis of sulfur-doped activated carbon, you must select your template size based on your chemical targets.
- If your primary focus is maximizing sulfur content: Utilize nano-magnesium oxide with a small particle size (ideally around 20 nm) to ensure maximum active surface area and reaction completeness.
- If your primary focus is avoiding process inefficiency: Reject larger particle templates, as they inherently restrict reaction space and will fail to achieve high doping percentages.
By prioritizing the smallest viable template size, you unlock the full chemical potential of the sulfur-carbon reaction.
Summary Table:
| Particle Size | Sample Identifier | Sulfur Doping Content | Reaction Efficiency |
|---|---|---|---|
| 20 nm | ACS-20 | 3.54% | High (Maximum active surface area) |
| Large (>20 nm) | Standard Templates | Low | Low (Limited reaction space) |
Unlock High-Performance Material Synthesis with KINTEK
Precise material engineering requires equipment that can handle demanding chemical processes. KINTEK provides industry-leading Muffle, Tube, Rotary, Vacuum, and CVD systems, all customizable to meet your specific research and synthesis needs.
Backed by expert R&D and world-class manufacturing, our lab high-temperature furnaces ensure the uniform heating and stability necessary for optimizing activated carbon doping and other advanced material applications.
Ready to elevate your research efficiency? Contact KINTEK today for a custom furnace solution!
Visual Guide
References
- Yaoping Guo, Rui Fang. Sulfur-doped activated carbon for the efficient degradation of tetracycline with persulfate: Insight into the effect of pore structure on catalytic performance. DOI: 10.1039/d3ra08958d
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Magnesium Extraction and Purification Condensing Tube Furnace
- Vacuum Dental Porcelain Sintering Furnace for Dental Laboratories
- Chairside Dental Porcelain Zirconia Sintering Furnace with Transformer for Ceramic Restorations
- 915MHz MPCVD Diamond Machine Microwave Plasma Chemical Vapor Deposition System Reactor
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
People Also Ask
- What are the equipment requirements for o-LISO ceramic sintering? Achieve 1050°C Precision for High Conductivity
- What role does a closed pressure vessel play during the carbonation of gamma-C2S? Unlock Rapid Mineralization
- Why is argon particularly attractive for industrial applications? Unlock Cost-Effective Purity and Stability
- Why are graphite molds preheated to 800 °C for Invar 36 casting? Unlock High-Quality Ingot Production
- What happens during the recovery stage of the annealing process? Unlock Stress Relief and Material Restoration
- What is the mechanism of solution treatment on Cu-Cr-Zr-La alloys? Master the Thermal Cycle for High-Strength Alloys
- Why is a pre-melting process required in phase equilibrium studies? Reset Your Sample for Precise Results
- Why is a laboratory constant temperature drying oven necessary for biomass adsorbents? Ensure Precision & Integrity



















