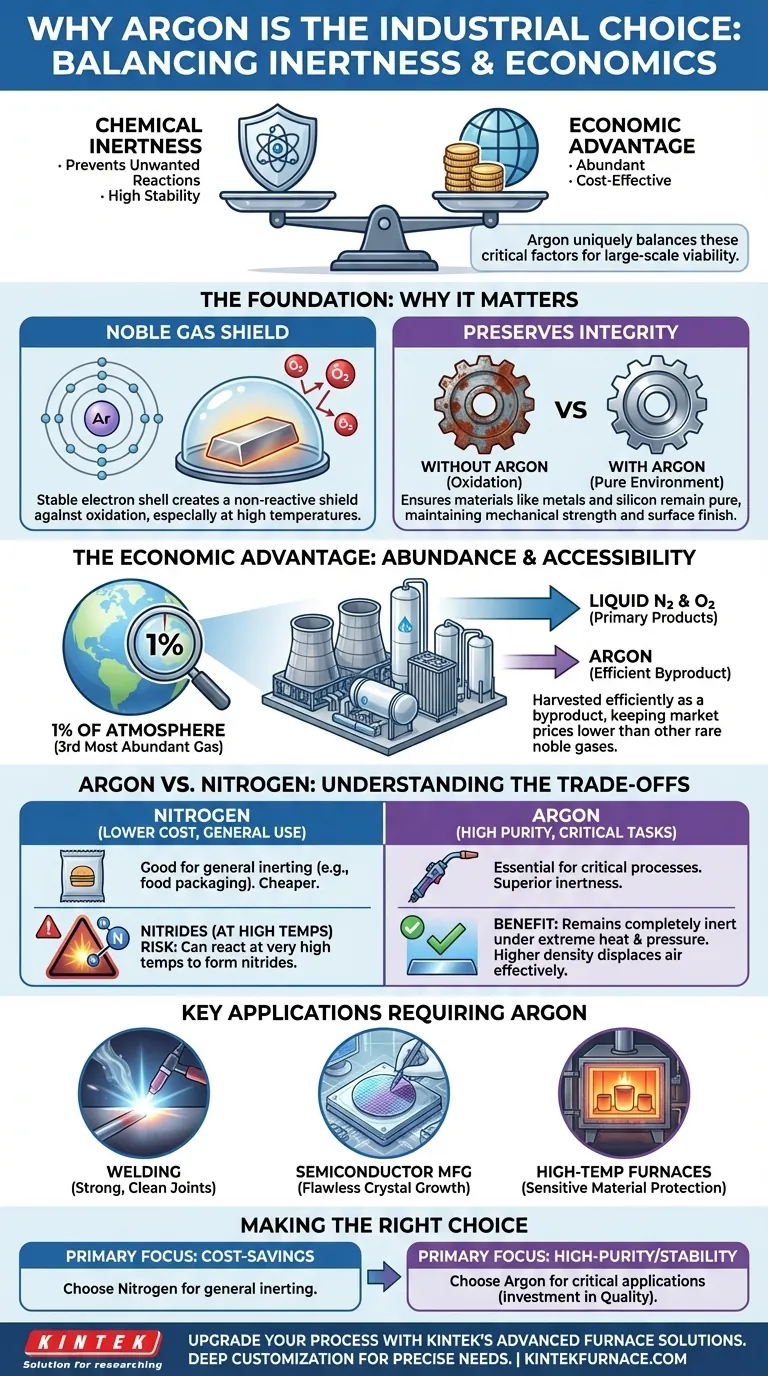
In short, argon is uniquely attractive for industrial applications because it perfectly balances two critical factors: it is chemically inert, preventing unwanted reactions, and it is abundant and cost-effective to produce, making it economically viable for large-scale use.
The core challenge in many high-temperature or high-purity industrial processes is protecting materials from chemical contamination, like oxidation. Argon provides an ideal solution by offering a superior inert shield at a cost that is far lower than other noble gases.

The Foundation: Why Chemical Inertness Matters
The primary value of argon comes from a fundamental chemical property: it is a noble gas. This means its outermost electron shell is full, making it extremely stable and non-reactive.
A Protective Gaseous Shield
In practice, this inertness allows argon to act as a protective shield. When flooded into an environment, it displaces reactive gases like oxygen.
This prevents oxidation and other unwanted chemical reactions that can degrade materials, especially at the high temperatures common in industrial processes.
Preserving Material Integrity
By preventing these reactions, an argon atmosphere ensures that materials like metals or silicon remain pure and uncontaminated.
This preserves their intended mechanical strength, chemical purity, and surface finish, which is critical in precision manufacturing.
The Economic Advantage: Abundance and Accessibility
While other noble gases are also inert, argon's widespread use is cemented by its favorable economics. It is not rare or prohibitively expensive.
An Abundant Atmospheric Resource
Argon is the third most abundant gas in Earth's atmosphere, making up nearly 1% of the air we breathe. This natural availability is the starting point for its cost-effectiveness.
A Byproduct of Air Separation
Industrially, argon is not produced on its own. It is harvested as a byproduct from cryogenic air separation units that are primarily designed to produce liquid nitrogen and liquid oxygen.
This symbiotic production process makes its extraction highly efficient and keeps its market price relatively low compared to other, rarer noble gases.
Understanding the Trade-offs: Argon vs. Nitrogen
The most common alternative for creating an inert atmosphere is nitrogen. Understanding when to choose argon over the less expensive nitrogen is key.
When Nitrogen Is Sufficient
Nitrogen is also relatively inert and is cheaper than argon. For many general-purpose applications, such as preventing basic oxidation in food packaging or some heat treatments, nitrogen is a perfectly adequate and more economical choice.
Why Argon Is Superior for Critical Tasks
However, at very high temperatures, nitrogen can become reactive and form unwanted compounds called nitrides with certain metals.
Argon remains completely inert even under extreme heat and pressure. This makes it the only safe choice for processes where even minimal contamination is unacceptable. Its higher density also makes it more effective at displacing ambient air in open-area applications like welding.
Key Applications Requiring Argon
This superior stability is why argon is mandated for applications like:
- Welding: It shields the molten weld pool from oxygen and nitrogen, preventing porosity and ensuring a strong, clean joint.
- Semiconductor Manufacturing: It provides a perfectly pure environment necessary for growing flawless silicon crystals.
- High-Temperature Furnaces: It protects sensitive metals from reacting with trace elements during processing.
Making the Right Choice for Your Process
Your choice of an inert gas depends entirely on the sensitivity of your application and your budget.
- If your primary focus is cost-savings for general inerting: Nitrogen is often the most economical choice for preventing basic oxidation where material purity is not the absolute top priority.
- If your primary focus is high-purity processing or high-temperature stability: Argon's superior inertness is non-negotiable for guaranteeing material integrity in critical applications like TIG welding or semiconductor fabrication.
Ultimately, choosing argon is an investment in process stability and final product quality.
Summary Table:
| Aspect | Details |
|---|---|
| Chemical Property | Noble gas, inert, non-reactive, prevents oxidation and contamination |
| Economic Advantage | Abundant in atmosphere (1%), cost-effective as a byproduct of air separation |
| Key Applications | Welding, semiconductor manufacturing, high-temperature furnaces |
| Comparison with Nitrogen | Superior for high-temperature stability; nitrogen is cheaper but may form nitrides |
Upgrade your industrial processes with KINTEK's advanced furnace solutions! Leveraging exceptional R&D and in-house manufacturing, we provide diverse laboratories with high-temperature furnaces like Muffle, Tube, Rotary, Vacuum & Atmosphere, and CVD/PECVD Systems. Our deep customization capability ensures precise fit for your unique needs, enhancing purity and efficiency. Contact us today to discuss how we can support your critical applications with reliable, tailored solutions!
Visual Guide
Related Products
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
People Also Ask
- What is an atmosphere protection muffle furnace? Unlock Precise Heat Treatment in Controlled Environments
- How do argon and nitrogen protect samples in vacuum furnaces? Optimize Your Thermal Process with the Right Gas
- How does a mixed gas flow control system maintain stability during high-temperature nitriding? Precision Gas Ratios
- What are some specific applications of atmosphere furnaces in the ceramics industry? Enhance Purity and Performance
- How does the pressure range change under vacuum conditions in an atmosphere box furnace? Explore Key Shifts for Material Processing



















