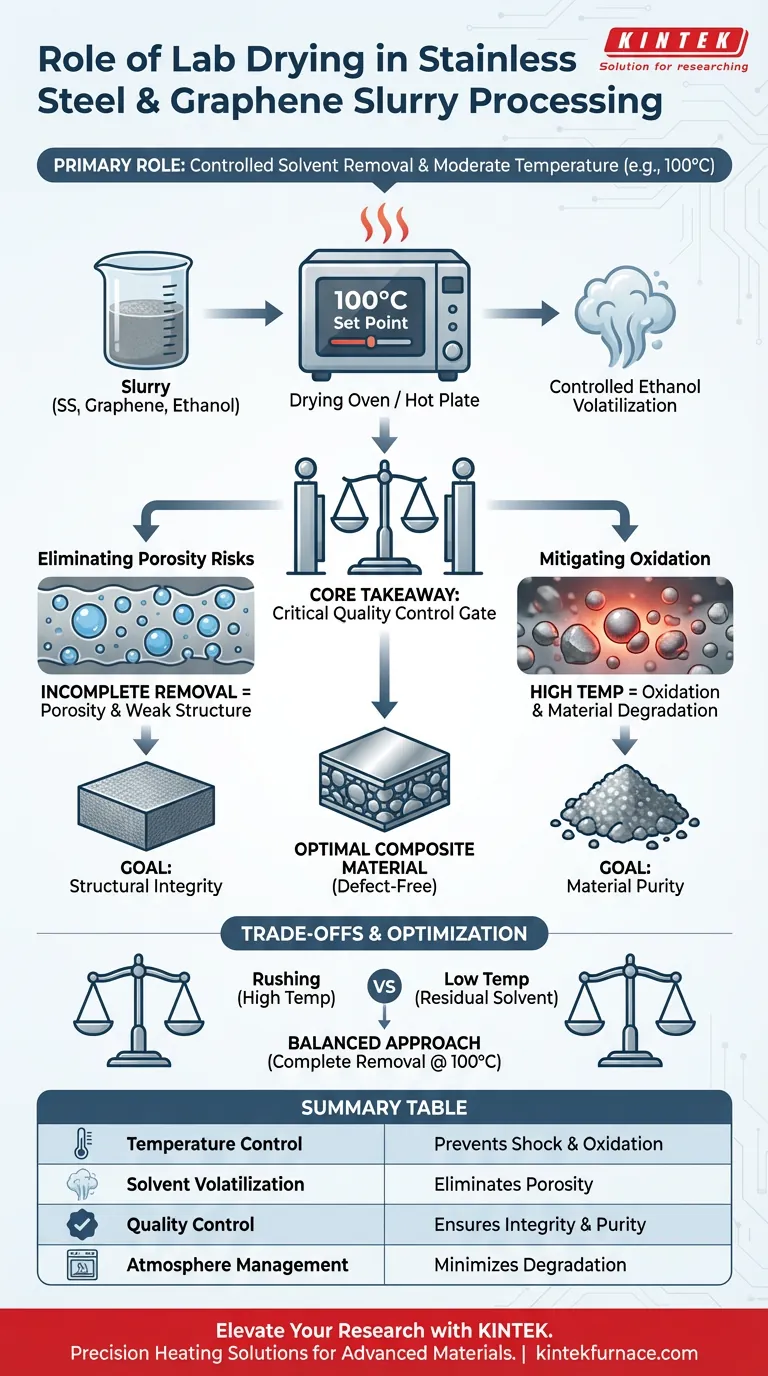
The primary role of a laboratory drying oven or hot plate is to facilitate the controlled removal of liquid solvents, typically ethanol, from the stainless steel and graphene slurry following wet mixing. By maintaining a constant, moderate temperature (such as 100 °C), the equipment ensures the solvent volatilizes completely without thermally damaging the composite components.
Core Takeaway: Controlled drying acts as a critical quality control gate. It ensures the total removal of solvents to prevent structural defects like porosity, while strictly limiting temperature to prevent the oxidation of the stainless steel powder.

The Mechanics of Controlled Drying
Ensuring Complete Volatilization
The immediate goal of the drying stage is the total removal of the liquid medium used during mixing.
In this specific process, ethanol is used as the solvent. The heating device is set to a temperature high enough to drive off the ethanol efficiently, ensuring no liquid remains in the mixture.
Regulating Thermal Input
Precision is key; the device does not simply apply heat, but maintains a specific set point, often 100 °C.
This constant temperature provides enough energy to evaporate the solvent without subjecting the materials to thermal shock or uncontrolled spikes in heat.
Preventing Downstream Defects
Eliminating Porosity Risks
If the drying process is incomplete, residual solvents remain trapped within the composite powder.
During the subsequent pressing or compaction stages, these trapped solvents create voids. This leads to the formation of pores, which significantly weakens the structural integrity of the final composite material.
Mitigating Oxidation
Stainless steel powder is susceptible to oxidation if exposed to excessive temperatures in an oxygen-rich environment.
By capping the temperature at a moderate level (e.g., 100 °C), the drying oven or hot plate removes moisture without reaching the activation energy required for significant metal oxidation.
Understanding the Trade-offs
The Risk of Rushing the Process
Attempting to speed up drying by increasing the temperature above the recommended set point is a common pitfall.
While this may remove the solvent faster, it drastically increases the likelihood of oxidizing the metal powder, degrading the material's properties before it is even formed.
The Consequence of Low Temperatures
Conversely, setting the temperature too low to preserve the material can result in incomplete drying.
This leaves residual ethanol in the slurry, which inevitably causes porosity defects during the pressing phase, rendering the part mechanically unsound.
Optimizing Your Processing Strategy
To ensure the highest quality stainless steel and graphene composite, align your drying approach with your specific material goals:
- If your primary focus is Structural Integrity: Prioritize complete volatilization of the ethanol to eliminate the risk of porosity during pressing.
- If your primary focus is Material Purity: Strictly maintain the temperature at or near 100 °C to prevent the oxidation of the stainless steel powder.
Balancing complete solvent removal with moderate temperature control is the definition of successful slurry processing.
Summary Table:
| Feature | Role in Slurry Processing | Impact on Final Composite |
|---|---|---|
| Temperature Control | Maintains constant set point (e.g., 100°C) | Prevents thermal shock and oxidation |
| Solvent Volatilization | Facilitates complete removal of ethanol | Eliminates porosity and structural voids |
| Quality Control | Acts as a gate before pressing/compaction | Ensures material purity and integrity |
| Atmosphere Management | Controlled heating in oven environment | Minimizes metal degradation and oxidation |
Elevate Your Composite Material Research with KINTEK
Precision heating is the foundation of high-performance materials. At KINTEK, we understand that even the drying phase is critical to your success. Backed by expert R&D and world-class manufacturing, we provide high-precision Laboratory Ovens, Muffle, Tube, and Vacuum systems designed to give you absolute control over your thermal processing.
Whether you are developing stainless steel composites or advanced graphene materials, our systems are fully customizable to meet your unique research needs and prevent defects like oxidation or porosity.
Ready to optimize your lab's efficiency? Contact KINTEK today to find the perfect heating solution for your application.
Visual Guide
References
- Kalyanamanohar Veeramallu, Alluru Gopala Krishna. Enhanced Wear and Corrosion Performance of Stainless Steel 316L with Addition of Different Weight Percentages of GNP. DOI: 10.62753/ctp.2024.04.1.1
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1200℃ Muffle Oven Furnace for Laboratory
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- Vacuum Hot Press Furnace Machine Heated Vacuum Press Tube Furnace
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
People Also Ask
- What are the primary uses of a muffle furnace? Achieve Precise High-Temperature Processing
- How do a laboratory high-temperature box furnace and water quenching work together? Optimize High-Manganese Steel
- What are the technical specifications of typical muffle furnaces? Key Specs for Precise Thermal Processing
- What are some common applications of muffle furnaces? Unlock Clean, High-Temperature Solutions for Your Lab
- Why is a low-temperature annealing furnace necessary for coal tar film sensor production? Achieve Precise Stabilization
- What are the benefits of front loading furnaces? Boost Efficiency and Safety in Your Lab
- How to calibrate a muffle furnace? Ensure Precise Temperature Control for Your Lab
- What are the advantages of using a laboratory infrared heating furnace for PET waste conversion? Boost Energy Efficiency



















