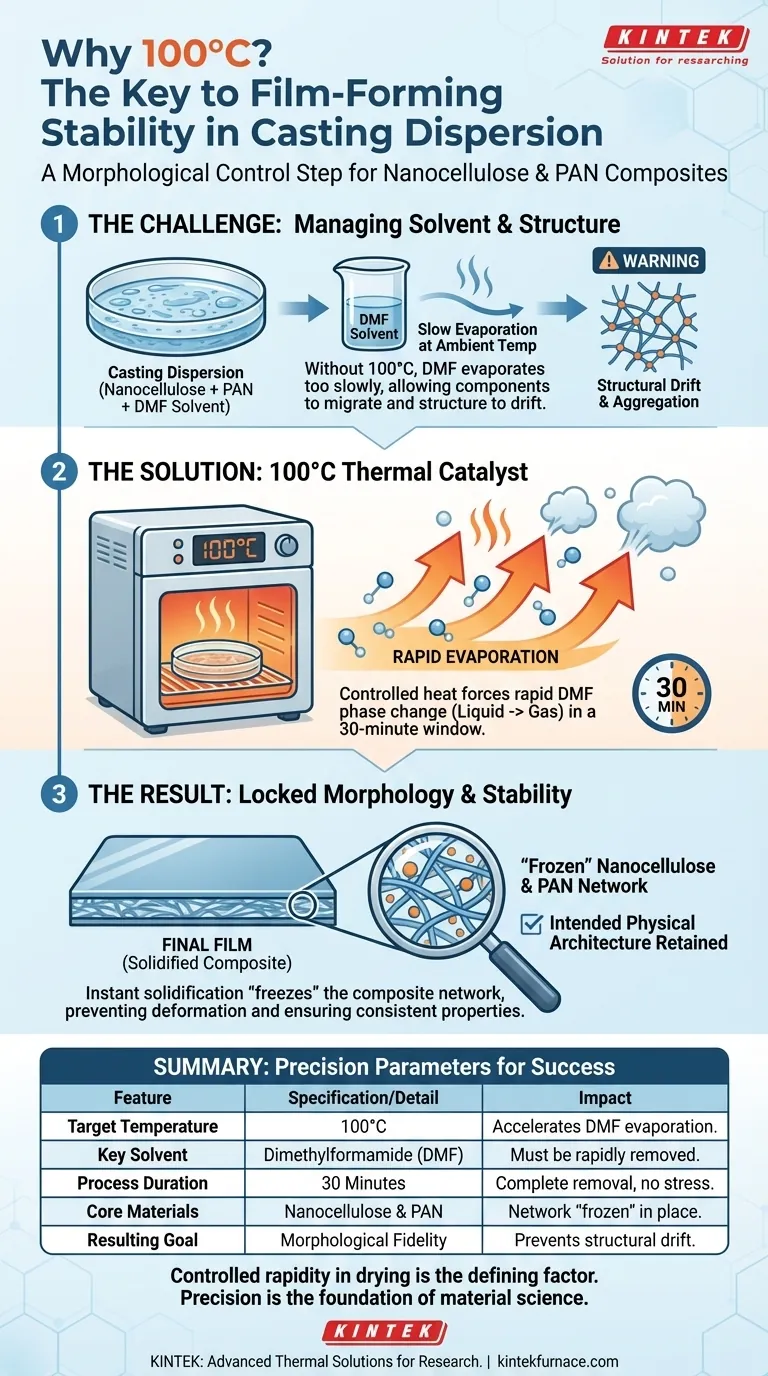
The application of controlled heat is the catalyst for structural stability. The casting dispersion must be treated in a 100°C drying oven to force the rapid evaporation of the organic solvent, dimethylformamide (DMF). This thermal acceleration causes the solute to solidify quickly, ensuring the final film retains the specific physical architecture intended for the application.
Core Takeaway The 100°C treatment is a morphological control step, not just a drying method. By rapidly driving off the DMF solvent, you effectively "freeze" the nanocellulose and polyacrylonitrile (PAN) composite network in place, preventing the structural deformation that occurs during slow, ambient drying.

The Mechanics of Solvent Evaporation
Overcoming Solvent Characteristics
The process relies on the removal of dimethylformamide (DMF), an organic solvent that must be evacuated from the mixture efficiently.
Placing the petri dish in a 100°C environment provides the controlled heat necessary to accelerate the phase change of DMF from liquid to gas.
Without this elevated temperature, the solvent would evaporate too slowly, leaving the dispersion in a liquid state for an extended period.
Rapid Solidification
The primary objective of this 30-minute thermal cycle is to make the solute solidify and deposit into a film instantly.
Speed is a critical variable here; the transition from dispersion to solid film must happen quickly to capture the material's properties.
This rapid deposition prevents the components from settling or separating, which can happen in lower-temperature environments.
Preserving Material Morphology
Stabilizing the Composite Network
The film is composed of a complex network of nanocellulose and polyacrylonitrile (PAN).
The interaction between these two materials dictates the physical properties of the final film.
The 100°C treatment ensures that this composite network maintains its intended physical morphology throughout the drying process.
Preventing Structural Drift
If the solvent is removed slowly, the internal structure of the composite has time to shift.
Rapid evaporation locks the specific arrangement of the nanocellulose and PAN in place.
This guarantees that the physical structure formed in the dispersion carries over accurately to the dry film.
Understanding the Trade-offs
The Consequence of Low Temperature
If the drying temperature drops significantly below 100°C, the evaporation rate of DMF decreases.
This extended drying time allows the nanocellulose and PAN components to migrate, potentially leading to aggregation or a loss of the desired network structure.
The Necessity of Time Control
While heat is vital, the duration is also specific; the reference cites a 30-minute window.
This duration is calculated to ensure complete solvent removal without subjecting the formed film to unnecessary thermal stress after the DMF is gone.
Making the Right Choice for Your Goal
To replicate the desired material properties, you must view temperature as a structural tool.
- If your primary focus is Morphological Fidelity: Maintain a strict 100°C environment to "lock" the nanocellulose and PAN network immediately upon casting.
- If your primary focus is Solvent Elimination: Ensure the full 30-minute cycle is completed to fully drive off the DMF, as residual solvent will compromise the film's solid state.
Controlled rapidity in drying is the defining factor between a successful composite film and a failed experiment.
Summary Table:
| Feature | Specification/Detail | Impact on Film Quality |
|---|---|---|
| Target Temperature | 100°C | Accelerates DMF solvent evaporation and phase change. |
| Key Solvent | Dimethylformamide (DMF) | Must be rapidly removed to prevent solute migration. |
| Process Duration | 30 Minutes | Ensures complete solvent removal without thermal stress. |
| Core Materials | Nanocellulose & PAN | Rapid drying 'freezes' the composite network in place. |
| Resulting Goal | Morphological Fidelity | Prevents structural drift, aggregation, and deformation. |
Precision is the foundation of material science. KINTEK provides the advanced thermal solutions necessary to achieve perfect morphological fidelity in your research. Backed by expert R&D and manufacturing, we offer high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems, all fully customizable for your unique lab requirements. Whether you are stabilizing nanocellulose composites or optimizing solvent evaporation, our high-temp furnaces ensure consistent results every time. Contact KINTEK today to discuss your custom furnace needs!
Visual Guide
References
- Suman, Bharat Bajaj. Low-Temperature Carbonization of Phosphorus-Doped Nanocellulose for Carbon Nanofiber Film Fabrication. DOI: 10.1007/s11837-024-07098-w
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1200℃ Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- Vacuum Dental Porcelain Sintering Furnace for Dental Laboratories
People Also Ask
- Why is a precision electric heating reactor used for ozone treatment of porous graphene? Unlock Angstrom-Scale Accuracy
- How does a high-sensitivity non-contact microphone assist in detecting cracks during the solidification of molten slag?
- What is the role of a sealed heating reactor in MSNs synthesis? Master Precision Pore Uniformity
- How is an industrial heating furnace used for 20MnCr gear steel normalization? Master Microstructural Integrity
- What is the purpose of preheating metal molds? Enhance Fluidity and Quality in Aluminum-Lithium Squeeze Casting
- Why is staged debinding necessary for perovskite ceramic green bodies? Prevent Cracking with Precision Control
- What is the primary role of a carbonization curing chamber? Unlock High Strength in Magnesium Slag Mortar
- What is the function of a laboratory cryofurnace during Co3O2BO3 experiments? Precise Phase Transition Control



















