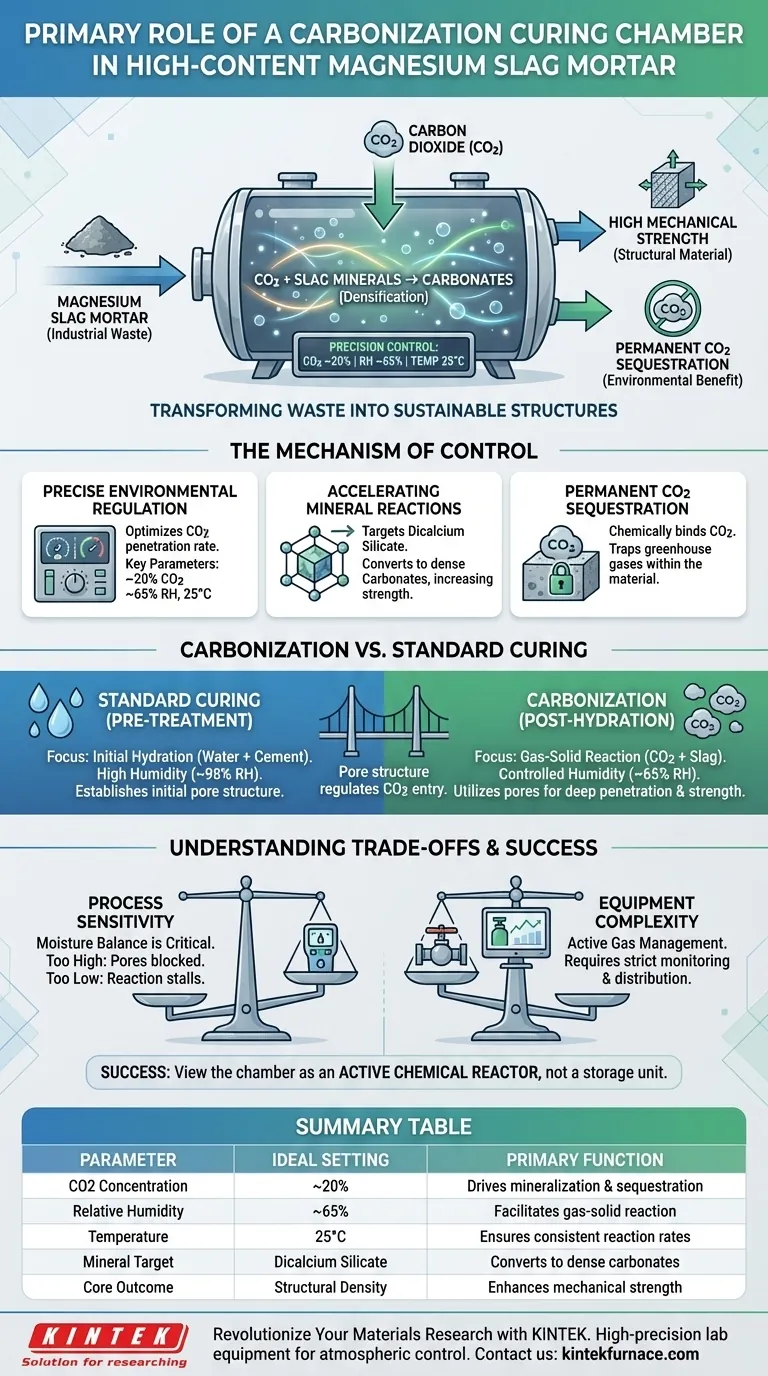
The primary role of a carbonization curing chamber is to facilitate and accelerate the chemical reaction between magnesium slag minerals and carbon dioxide by maintaining a strictly controlled environment. By regulating critical parameters such as CO2 concentration, relative humidity, and temperature, the chamber ensures the mortar achieves high mechanical strength while permanently sequestering carbon dioxide.
The chamber acts as a chemical reactor that transforms industrial waste into a structural material. It shifts the curing process from simple hydration to active mineralization, enhancing both the material's durability and its environmental footprint.

The Mechanism of Carbonization Control
Precise Environmental Regulation
The effectiveness of the carbonization curing chamber relies on its ability to maintain specific atmospheric conditions that differ significantly from standard ambient air.
Typically, the chamber maintains a CO2 concentration of approximately 20%, a relative humidity of around 65%, and a steady temperature of 25°C. These specific parameters are engineered to optimize the rate at which CO2 penetrates the mortar and reacts with the binder.
Accelerating Mineral Reactions
Inside the chamber, the controlled environment targets specific minerals present in the magnesium slag, such as dicalcium silicate.
The elevated CO2 concentration drives a reaction that converts these minerals into carbonates. This process densifies the microstructure of the mortar, directly contributing to its final mechanical strength.
Permanent CO2 Sequestration
Beyond structural integrity, the chamber serves an environmental function.
The reaction promoted within the chamber chemically binds CO2 into the solid phase of the material. This results in permanent carbon sequestration, effectively trapping greenhouse gases within the building material itself.
Distinguishing Carbonization from Standard Curing
The Role of Pre-Treatment
It is critical to distinguish the carbonization chamber from a standard constant temperature and humidity curing box.
Standard curing boxes usually maintain a very high humidity (e.g., 98% RH) to facilitate initial hydration. This pre-treatment step establishes the initial pore structure and strength of the matrix.
The Role of Carbonization
The carbonization chamber is utilized after the initial hydration phase.
While the standard box focuses on hydraulic reaction (water + cement), the carbonization chamber focuses on the gas-solid reaction (CO2 + slag). The pore structure formed during pre-treatment regulates how effectively CO2 can penetrate the material once it enters the carbonization chamber.
Understanding the Trade-offs
Process Sensitivity
Carbonization curing is highly sensitive to the moisture content within the mortar.
If the relative humidity in the carbonization chamber is too high, water molecules may block the pores, preventing CO2 from penetrating deep into the material. Conversely, if it is too low, the chemical reaction may stall for lack of a reaction medium.
Equipment Complexity
Unlike standard curing, which primarily requires moisture retention, carbonization requires active gas management.
Operators must strictly monitor CO2 levels and ensure the gas is evenly distributed. This adds a layer of operational complexity compared to traditional hydration curing methods.
Making the Right Choice for Your Goal
To optimize the preparation of high-content magnesium slag mortar, you must balance the initial hydration with the subsequent carbonization.
- If your primary focus is establishing the material structure: Prioritize the standard curing phase (98% RH) to build the initial pore network and strength needed for the material to hold its shape.
- If your primary focus is maximizing strength and carbon uptake: Ensure strict adherence to the carbonization chamber parameters (20% CO2, 65% RH) to drive the mineralization reaction to completion.
Success lies in viewing the carbonization chamber not as a storage unit, but as an active chemical reactor that defines the final properties of your material.
Summary Table:
| Parameter | Ideal Setting | Primary Function |
|---|---|---|
| CO2 Concentration | ~20% | Drives mineralization and carbon sequestration |
| Relative Humidity | ~65% | Facilitates gas-solid reaction without blocking pores |
| Temperature | 25°C | Ensures consistent chemical reaction rates |
| Mineral Target | Dicalcium Silicate | Converts waste minerals into dense carbonates |
| Core Outcome | Structural Density | Enhances mechanical strength and durability |
Revolutionize Your Materials Research with KINTEK
Are you looking to optimize carbon sequestration and material durability in your lab? Backed by expert R&D and manufacturing, KINTEK offers high-precision Muffle, Tube, Rotary, Vacuum, and CVD systems, alongside customizable lab high-temp furnaces tailored for unique research needs.
Whether you are developing sustainable magnesium slag mortar or advanced ceramics, our equipment provides the precise atmospheric control required for success. Contact us today to find the perfect solution for your research!
Visual Guide
References
- Gang Liu, Jianyun Wang. Effects of Hydraulic Materials on the Performance Evolution of Carbonated High-Volume Magnesium Slag Mortars. DOI: 10.3390/buildings15173062
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
People Also Ask
- What is the primary function of a drying oven in the preparation of calcium oxide from eggshell waste? Maximize Purity
- What is preventive maintenance on a furnace? A Proactive Strategy for Peak Performance
- What is the primary function of a high-temperature sintering furnace operating at 1173 K in the preparation of porous oxide precursors? Achieve Structural Integrity for Your Precursors
- What is the purpose of the annealing process in OLED preparation? Optimize Film Stability and Device Efficiency
- Why is vacuum impregnation necessary for PAN-GF electrodes? Ensure Peak Fiber Conductivity and Slurry Integration
- How does the introduction of SiO2 as an additive improve the sintering process of solid electrolytes? Boost Densification
- How does glass frit function in SiOC coatings? Enhance Barrier Density with Liquid-Phase Healing
- How does a symmetric suction design improve steel wire heat treatment? Achieve Uniform Salt Flow and Sorbite Quality



















