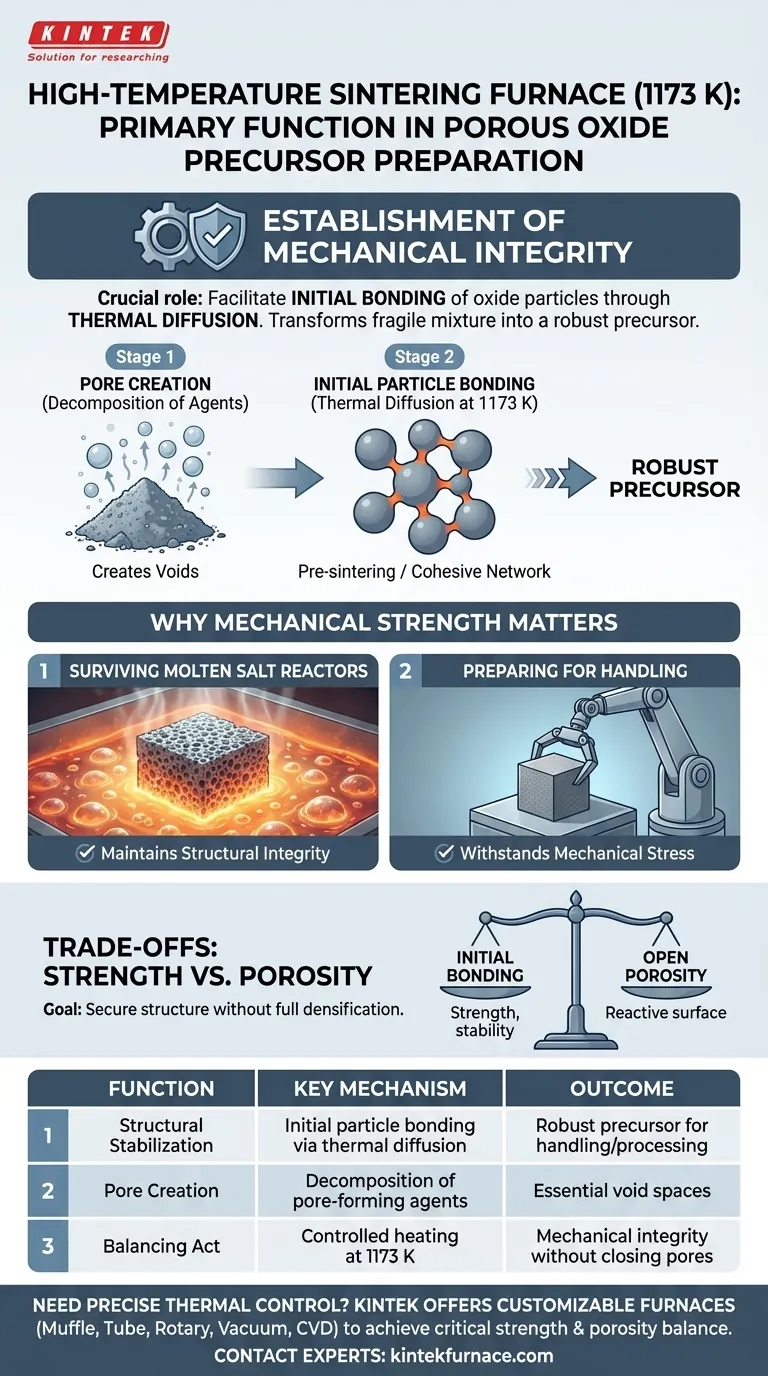
The primary function is the establishment of mechanical integrity. While the furnace at 1173 K does decompose pore-forming agents to create voids, its most critical role is to facilitate the initial bonding of oxide particles through thermal diffusion. This process transforms a fragile mixture into a robust precursor capable of surviving harsh downstream environments.
The core objective at this temperature is not full densification, but rather structural stabilization. The heat treatment imparts just enough mechanical strength to maintain the porous shape without closing off the essential void spaces.

The Mechanism of Precursor Formation
Beyond Pore Creation
It is a common misconception that the furnace is used solely to remove the pore-forming agent.
While the heat does decompose these agents to generate the desired porous structure, this is only the first step. If the process stopped here, the remaining oxide skeleton would be too fragile to handle.
Initial Particle Bonding
The defining function at 1173 K is thermal diffusion.
At this temperature, the oxide particles begin to bond with one another. This "pre-sintering" initiates the growth of necks between particles, creating a cohesive network rather than a loose pile of dust.
Why Mechanical Strength Matters
Surviving Molten Salt Reactors
The references highlight a specific downstream application: molten salt electrolysis.
The precursor must possess sufficient strength to maintain its structural integrity when submerged in a molten salt reactor. Without the bonding achieved at 1173 K, the porous oxide would likely disintegrate upon contact with the reactive, turbulent salt melt.
Preparing for Handling
This heating stage serves as a bridge between raw compaction and final usage.
Whether the next step involves high-pressure hot re-pressing or direct electrolysis, the "green compact" (the pressed powder) requires preliminary bonding to withstand mechanical stress. The furnace ensures the material is robust enough to be moved and processed without crumbling.
Understanding the Trade-offs
Strength vs. Porosity
There is a delicate balance to strike during this heat treatment phase.
The goal is to achieve initial bonding without triggering full sintering. If the temperature were significantly higher or maintained too long, the material might densify completely, closing the pores you worked to create.
Conversely, if the bonding is insufficient, the precursor will fail mechanically. The 1173 K operating point is selected to secure the structure while preserving the open porosity required for chemical interaction.
Making the Right Choice for Your Goal
To optimize your preparation of porous oxide precursors, consider the following regarding the sintering temperature:
- If your primary focus is Structural Survival: Ensure the residence time at 1173 K is sufficient to maximize thermal diffusion, preventing disintegration in the electrolysis reactor.
- If your primary focus is Pore Connectivity: Monitor the bonding process to ensure particle necking does not advance to the point of closing off the porous channels required for reaction efficiency.
The furnace ultimately acts as a stabilizer, locking in the porous architecture so it can perform its function in the electrolytic cell.
Summary Table:
| Function | Key Mechanism | Outcome |
|---|---|---|
| Structural Stabilization | Initial particle bonding via thermal diffusion | Robust precursor capable of handling and downstream processing |
| Pore Creation | Decomposition of pore-forming agents | Generation of essential void spaces and porous architecture |
| Balancing Act | Controlled heating at 1173 K | Achieves mechanical integrity without closing off pores |
Need a furnace that delivers precise thermal control for your porous precursor development?
Backed by expert R&D and manufacturing, KINTEK offers Muffle, Tube, Rotary, Vacuum, and CVD systems, and other lab high-temp furnaces, all customizable for unique needs like achieving the critical balance between mechanical strength and porosity. Our furnaces provide the reliable performance required for applications from materials research to molten salt electrolysis.
Contact our experts today to discuss how a KINTEK furnace can stabilize your process and enhance your results.
Visual Guide
Related Products
- Molybdenum Vacuum Heat Treat Furnace
- 1400℃ Muffle Oven Furnace for Laboratory
- Vacuum Heat Treat Sintering Furnace Molybdenum Wire Vacuum Sintering Furnace
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
People Also Ask
- What are the advantages of the sol-gel nitrate combustion method? Achieve Atomic-Level Purity in Oxide Synthesis
- What are the critical functions of cold recycled gas nozzles? Optimize Oil Shale Retorting and Energy Recovery
- How does a vacuum pressure infiltration system contribute to Diamond/Cu composite green bodies? Achieve 60% Density
- Why is precise temperature control in a vacuum drying oven critical for CoTe@Ti3C2 battery electrodes? Key Insights.
- Why are reactive polyurethane systems a focus of thermal analysis in leather finishing? Balance Safety and Aesthetics
- What is the purpose of magnetron sputtering in N-I-P CsPbBr3 detectors? Optimize Charge Transport & Stability
- What is the significance of the calcination process for LaOx-modified platinum-based catalysts? Unlocking Pure Activity
- Why are batch furnaces considered essential for certain applications? Achieve Precision and Flexibility in Heat Treatment



















