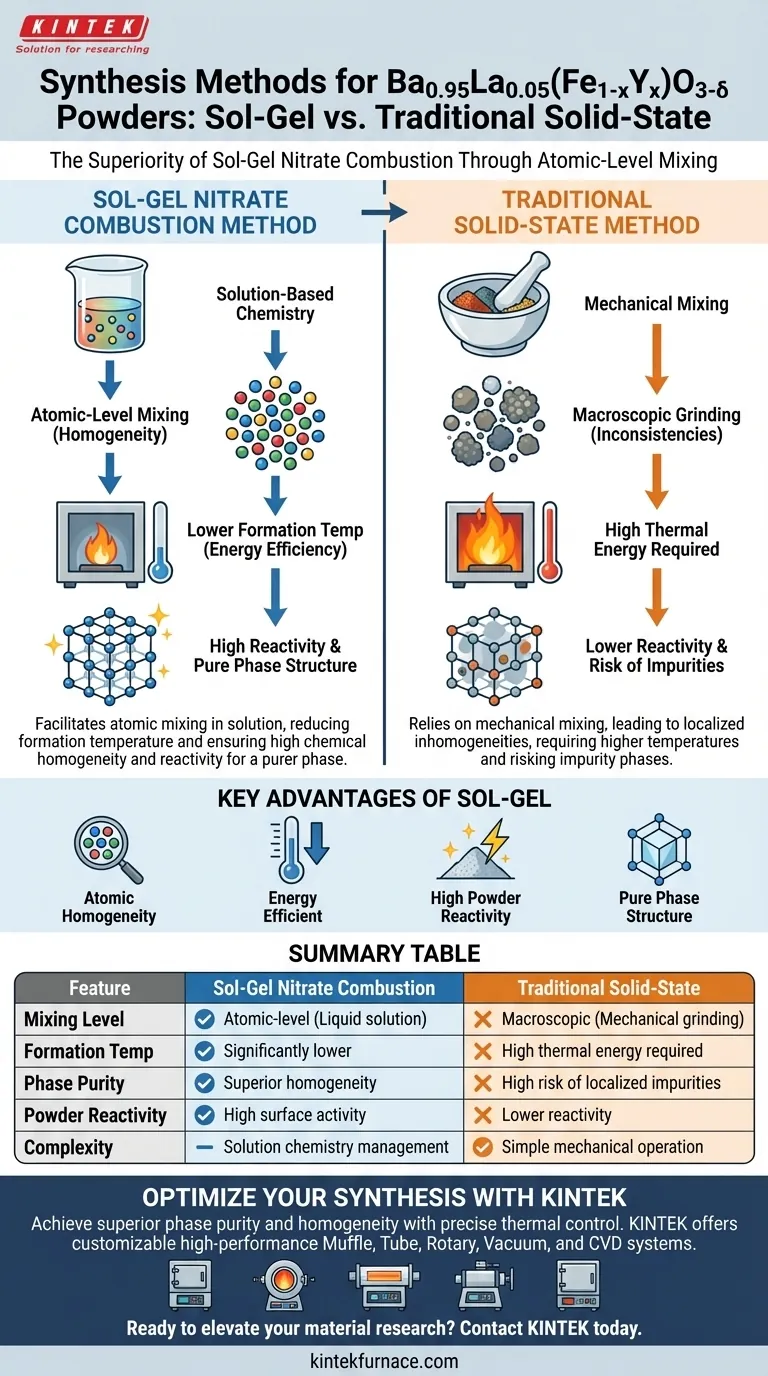
The primary advantage of the sol-gel nitrate combustion method over the traditional solid-state method lies in its ability to facilitate atomic-level mixing of the chemical components within a solution. This superior mixing significantly reduces the temperature required to form the Ba0.95La0.05(Fe1-xYx)O3-δ perovskite phase, while simultaneously ensuring higher chemical homogeneity and powder reactivity.
By shifting from mechanical mixing to solution-based chemistry, this method overcomes the diffusion limitations of solid-state reactions, delivering a purer phase structure with greater energy efficiency.

Achieving Homogeneity at the Source
Moving Beyond Mechanical Limits
Traditional solid-state synthesis relies on the mechanical mixing of powders. This often leads to localized inconsistencies where the ions are not perfectly distributed.
Atomic-Level Integration
The sol-gel nitrate combustion method resolves this by mixing components in a liquid solution. This ensures that the barium, lanthanum, iron, and yttrium ions are mixed at the atomic level before the combustion process even begins.
Thermal Efficiency and Phase Purity
Lowering Formation Temperatures
Because the components are already intimately mixed, less thermal energy is required to arrange them into the correct crystal lattice. Consequently, the formation temperature for the perovskite phase is significantly reduced compared to solid-state methods.
Enhancing Powder Reactivity
The powders produced via this combustion process exhibit higher reactivity. This increased surface activity creates a superior foundation for subsequent processing steps.
Securing a Pure Phase Structure
The combination of atomic mixing and high reactivity minimizes the risk of impurity phases. This provides a robust baseline for achieving a pure phase structure during the final heat treatments.
Operational Considerations
Complexity vs. Quality
While the solid-state method is often praised for its operational simplicity, it frequently compromises on uniformity. The sol-gel method creates a chemically superior product, but inherently involves managing solution chemistry rather than simple mechanical grinding.
Making the Right Choice for Your Project
To determine which method aligns with your synthesis goals, consider the following priorities:
- If your primary focus is maximum phase purity: Adopt the sol-gel nitrate combustion method to leverage atomic-level mixing and eliminate localized inhomogeneities.
- If your primary focus is energy efficiency: Choose the sol-gel method to take advantage of the significantly lower temperatures required for phase formation.
Ultimately, for the synthesis of complex oxides like Ba0.95La0.05(Fe1-xYx)O3-δ, solution-based combustion offers a distinct qualitative advantage over traditional solid-state techniques.
Summary Table:
| Feature | Sol-Gel Nitrate Combustion | Traditional Solid-State |
|---|---|---|
| Mixing Level | Atomic-level (Liquid solution) | Macroscopic (Mechanical grinding) |
| Formation Temp | Significantly lower | High thermal energy required |
| Phase Purity | Superior homogeneity | High risk of localized impurities |
| Powder Reactivity | High surface activity | Lower reactivity |
| Complexity | Solution chemistry management | Simple mechanical operation |
Optimize Your Synthesis Process with KINTEK
Transitioning to the sol-gel nitrate combustion method requires precise thermal control to achieve superior phase purity and homogeneity. KINTEK provides the cutting-edge laboratory equipment necessary to support your advanced chemical synthesis.
Backed by expert R&D and manufacturing, KINTEK offers high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems, all of which are fully customizable to meet the specific temperature profiles required for complex oxide production. Whether you are aiming for energy efficiency or atomic-level precision, our lab high-temp furnaces deliver the reliability you need.
Ready to elevate your material research? Contact KINTEK today to discuss your custom furnace solution!
Visual Guide
References
- Christian Berger, Rotraut Merkle. Ion transport in dry and hydrated Ba<sub>0.95</sub>La<sub>0.05</sub>(Fe<sub>1−<i>x</i></sub>Y<sub><i>x</i></sub>)O<sub>3−<i>δ</i></sub> and implications for oxygen electrode kinetics of protonic ceramic cells. DOI: 10.1039/d5ta03014e
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Laboratory Quartz Tube Furnace RTP Heating Tubular Furnace
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1400℃ Muffle Oven Furnace for Laboratory
- Multi Heating Zones CVD Tube Furnace Machine for Chemical Vapor Deposition Equipment
People Also Ask
- What are the advantages of using microwave plasma for aluminum powder reduction? Achieve Unmatched Material Purity
- Why is a mixture of Argon (Ar) and Hydrogen (H2) required during beryl heat treatment? Master Color Transformation
- How do continuous furnaces differ from batch furnaces? Choose the Right Furnace for Your Production Needs
- How does a benchtop industrial oven improve efficiency? Boost Energy Savings and Space Use
- What role does X-ray diffraction (XRD) play in evaluating ZIF thermal treatment? Master Material Transformation
- Why is a vibratory mill used for ultra-fine grinding when preparing magnesite samples for zeta potential tests?
- What is the primary purpose of operating a laboratory oven at 383 K for 24 hours? Precision Drying for Carbon Prep
- What is the function of industrial furnaces in 7075 aluminum solution treatment? Master Material Strength



















