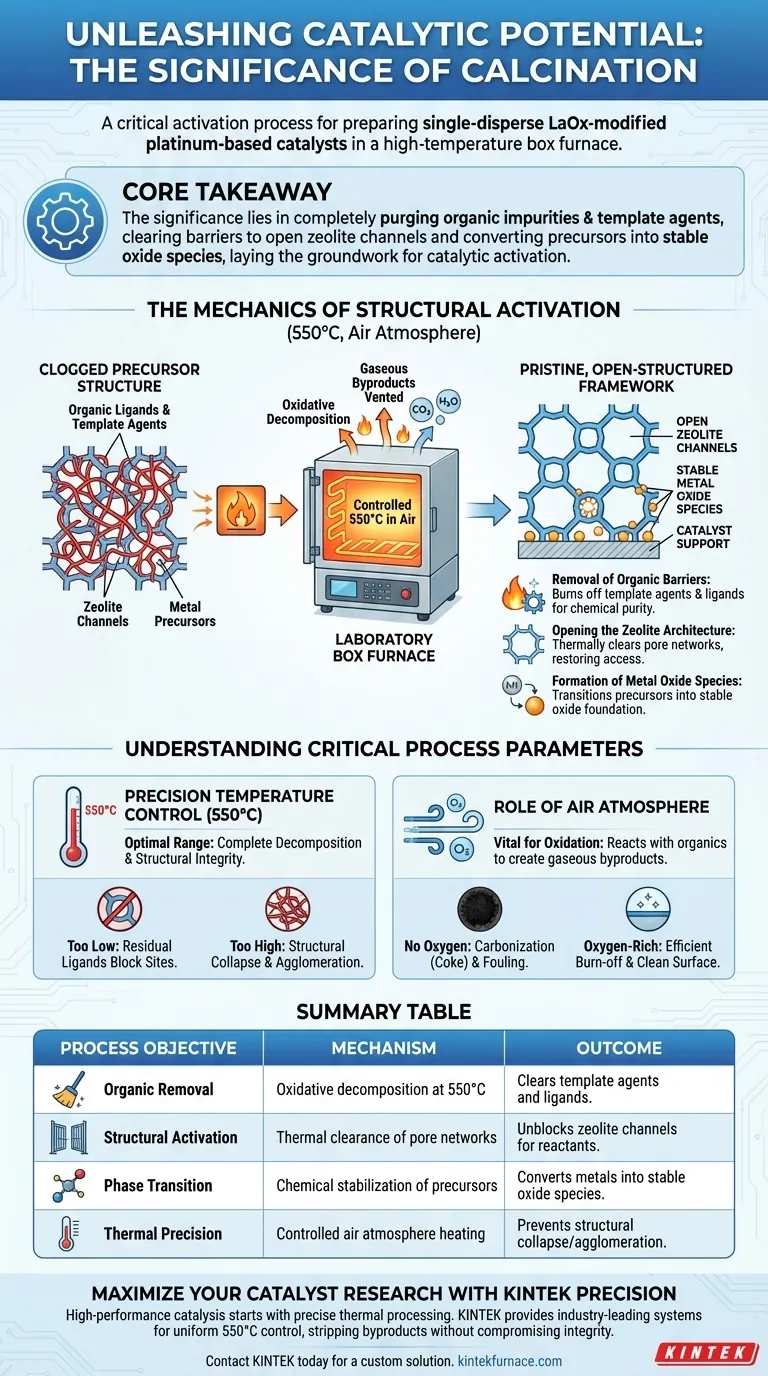
The laboratory high-temperature box furnace acts as the critical activation chamber for catalyst precursors. For single-disperse LaOx-modified platinum-based catalysts, this equipment is specifically used to perform calcination at 550 °C in an air atmosphere to strip away synthesis byproducts and prime the material's internal structure.
Core Takeaway The significance of this process lies in its ability to completely purge organic impurities and template agents that block active sites. By clearing these barriers, calcination opens the zeolite channels and converts metal precursors into stable oxide species, laying the essential groundwork for subsequent reduction and catalytic activation.

The Mechanics of Structural Activation
Removal of Organic Barriers
During synthesis, chemical agents such as template agents and organic ligands are used to direct the structure of the catalyst. However, these materials become liabilities in the final product.
The box furnace provides a controlled oxidative environment that burns these components off completely. This ensures that the final catalyst material is chemically pure and free of residual carbonaceous debris that could inhibit performance.
Opening the Zeolite Architecture
For catalysts involving zeolite structures, the internal pore network is the engine of chemical reactivity. Initially, this network is clogged by the very template agents used to build it.
Calcination effectively opens the zeolite channels. By thermally decomposing the blocking agents, the furnace restores the porous architecture, ensuring reactants can eventually access the internal surface area where the active sites reside.
Formation of Metal Oxide Species
Beyond cleaning the structure, calcination drives a fundamental chemical change. It transitions the metal components from their precursor state into initial metal oxide species.
This step stabilizes the metal species on the support. It creates a robust oxide foundation that is chemically ready to be converted into its final active metallic form during the subsequent reduction phase.
Understanding Critical Process Parameters
The Importance of Temperature Control
The specific target of 550 °C is not arbitrary. It is a precise thermal setpoint designed to be high enough to ensure the complete decomposition of organic ligands but controlled enough to avoid damaging the catalyst support.
If the temperature is too low, residual ligands (such as nitrates or acetylacetonates) may remain, blocking active sites. If uncontrolled, excessive heat could lead to the collapse of the zeolite structure or the unwanted agglomeration of metal particles.
The Role of Air Atmosphere
The presence of an air atmosphere is vital for the oxidation process. The oxygen in the air reacts with the organic templates and ligands, converting them into gaseous byproducts that are easily vented from the furnace.
Without this oxygen-rich environment, the organics would essentially carbonize (turn into coke) rather than burn off, permanently fouling the catalyst surface and blocking the zeolite channels.
Making the Right Choice for Your Goal
When configuring your calcination protocol for LaOx-modified platinum-based catalysts, consider the following priorities:
- If your primary focus is Pore Accessibility: Ensure the dwell time at 550 °C is sufficient to fully decompose the template agents, effectively unlocking the zeolite channels.
- If your primary focus is Active Site Stability: Verify that the air flow within the box furnace is consistent to facilitate the complete oxidation of precursors into their stable metal oxide forms before reduction.
Mastering the calcination step transforms a chemically clogged precursor into a pristine, open-structured framework ready for high-performance catalysis.
Summary Table:
| Process Objective | Mechanism | Outcome |
|---|---|---|
| Organic Removal | Oxidative decomposition at 550°C | Clears template agents and ligands |
| Structural Activation | Thermal clearance of pore networks | Unblocks zeolite channels for reactants |
| Phase Transition | Chemical stabilization of precursors | Converts metals into stable oxide species |
| Thermal Precision | Controlled air atmosphere heating | Prevents structural collapse/agglomeration |
Maximize Your Catalyst Research with KINTEK Precision
High-performance catalysis starts with precise thermal processing. KINTEK provides industry-leading Muffle, Tube, Rotary, Vacuum, and CVD systems designed to meet the rigorous demands of laboratory research.
Our high-temperature furnaces ensure the uniform 550°C air-atmosphere control essential for stripping synthesis byproducts and opening zeolite architectures without compromising material integrity. Backed by expert R&D and manufacturing, we offer fully customizable solutions tailored to your unique catalyst synthesis needs.
Ready to elevate your material activation? Contact KINTEK today for a custom solution.
Visual Guide
References
- Guilin Wei, Xingwen Feng. Embedding Monodisperse LaO <i> <sub>x</sub> </i> Into Pt Nanoclusters for Ultra‐Stable and Efficient Hydrogen Isotope Oxidation. DOI: 10.1002/advs.202504224
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- Laboratory Muffle Oven Furnace with Bottom Lifting
People Also Ask
- Why is the drying step using an industrial electric oven critical in catalyst preparation? Ensure Structural Integrity
- Why is a precision oven used to dry washed cherry pits? Unlock Superior Activated Carbon Production
- How does a precision temperature-controlled furnace facilitate the long-term aging treatment of Invar 36?
- How does the catalytic steam reforming system convert refinery waste gas into syngas for SOFC? Maximize Waste Energy
- Why is a laboratory vacuum drying oven essential for the swelling-encapsulation-shrinkage method? Lock-in Film Quality
- How are impurity levels controlled during tantalum powder synthesis? Master High-Purity Magnesiothermic Reduction
- Why is substrate preheating typically employed during the LPBF process? Minimize Stress & Prevent Cracks in 3D Printing
- What is the function of a laboratory high-temperature furnace in eggshell powder pretreatment? Optimize AA6061 Composites



















