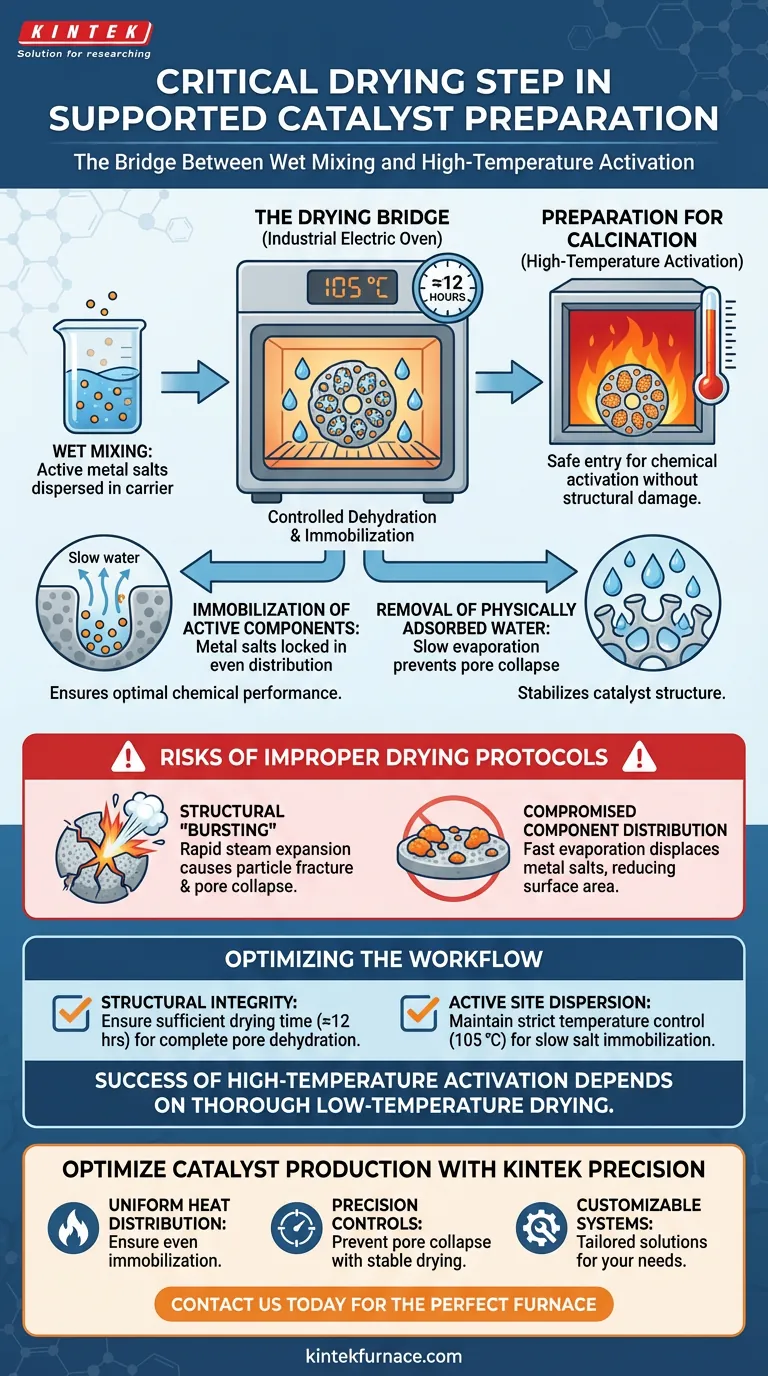
The drying step serves as the critical bridge between wet mixing and high-temperature activation. Using an industrial electric oven effectively immobilizes active metal salts on the carrier surface and removes moisture at a controlled rate. Without this specific thermal treatment, the catalyst’s physical structure would be compromised during the subsequent calcination phase.
By maintaining a steady temperature of 105 °C, the drying process slowly evaporates physically adsorbed water from within the catalyst pores. This controlled dehydration stabilizes the catalyst structure, preventing the catastrophic pore collapse and particle bursting that occur when retained moisture turns into rapidly expanding steam during high-temperature calcination.
The Mechanics of Controlled Drying
Immobilization of Active Components
During the wet mixing process, active metal salts are dispersed throughout the carrier. The drying step is essential to immobilize these salts on the surface of the support.
By removing the solvent (water) slowly, the active components are locked into position. This ensures an even distribution of the catalytic material, which is a prerequisite for optimal chemical performance.
Removal of Physically Adsorbed Water
Catalyst supports are highly porous, and water can become trapped deep within these microstructures. An industrial electric oven, typically set to 105 °C, targets this physically adsorbed water.
This temperature is sufficient to induce evaporation without triggering premature chemical reactions or thermal shock. The standard duration, often around 12 hours, ensures complete dehydration of the pore network.
Preparing for Calcination
The drying phase is effectively a safety measure for the subsequent calcination step. Calcination involves extremely high temperatures intended to chemically activate the catalyst.
If the catalyst is not thoroughly dried first, it enters calcination with significant moisture content. This moisture is the primary variable that determines whether the catalyst structure survives the final heating process.
Risks of Improper Drying Protocols
Structural "Bursting"
If a wet catalyst is exposed immediately to high calcination temperatures, the trapped water vaporizes instantly. The volume of water expands rapidly as it turns to steam.
This internal pressure can cause the bursting of catalyst particles or the collapse of the pore structure. The drying step mitigates this by removing the water gently before high heat is applied.
Compromised Component Distribution
Rapid moisture vaporization does more than just damage the support; it can also disrupt the active metals.
Fast evaporation can displace the metal salts, leading to uneven distribution or clumping. This lack of uniformity significantly reduces the surface area available for reactions, degrading the final efficiency of the catalyst.
Optimizing the Catalyst Preparation Workflow
To ensure the physical stability and chemical activity of your supported catalysts, consider these focal points:
- If your primary focus is Structural Integrity: Ensure the drying cycle is sufficiently long (typically 12 hours) to remove all pore-bound moisture, preventing steam-induced fracturing during calcination.
- If your primary focus is Active Site Dispersion: Maintain a strict temperature control of 105 °C to immobilize metal salts slowly, preventing the migration or aggregation of active components.
The success of high-temperature activation is entirely dependent on the thoroughness of low-temperature drying.
Summary Table:
| Drying Factor | Process Impact | Critical Benefit |
|---|---|---|
| Temperature (105°C) | Slow moisture evaporation | Prevents steam expansion & particle bursting |
| Solvent Removal | Immobilizes metal salts | Ensures uniform distribution of active components |
| Standard Duration | Deep pore dehydration | Prepares the support for high-heat calcination |
| Atmosphere Control | Controlled dehydration | Protects the porous microstructure from collapse |
Optimize Your Catalyst Production with KINTEK Precision
Don't let improper drying compromise your catalyst's efficiency. KINTEK provides industry-leading thermal solutions designed for the rigorous demands of catalyst preparation. Backed by expert R&D and manufacturing, we offer high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems, all customizable for your unique laboratory or industrial needs.
Our value to you:
- Uniform Heat Distribution: Ensure even immobilization of active metal salts.
- Precision Controls: Prevent pore collapse with stable, low-temperature drying protocols.
- Customizable Systems: Tailored solutions to match your specific carrier and salt chemistry.
Ready to enhance your material stability? Contact us today to find the perfect furnace for your application!
Visual Guide
References
- Darzhan Aitbekova, Т. О. Хамитова. The Use of the Catalysts Based on Coal Ash Microsphere and Chrysotile in the Thermal Destruction of Primary Coal Tar. DOI: 10.31489/2959-0663/1-24-9
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1200℃ Muffle Oven Furnace for Laboratory
- Electric Rotary Kiln Continuous Working Small Rotary Furnace Kiln for Pyrolysis Plant Heating
- Electric Rotary Kiln Pyrolysis Furnace Plant Machine Small Rotary Kiln Calciner
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
People Also Ask
- Why must the casting dispersion be treated in a 100°C drying oven? Ensure Perfect Film Morphology
- Why is it necessary to preheat casting molds to 300°C? Expert Thermal Control for Recycled Aluminum Alloy Production
- What are the advantages of combining vacuum hot rolling with small hole vacuuming? High-Bonding Clad Plate Production
- What are the applications of heat treatment furnaces in the aerospace industry? Enhance Component Performance for Extreme Conditions
- Why is a slow heating rate utilized for rice husk biochar? Optimize Pore Structure and Adsorption Performance
- Why is Boron Nitride (BN) powder used as a diluent? Enhance Accuracy in Iron Oxidation Kinetics
- What are the advantages of ascorbic acid over glucose in LFP synthesis? Achieve Superior Purity and Crystallinity
- What are the technical functions of carrier gases in VTD? Master Vapor Transport Deposition Control



















