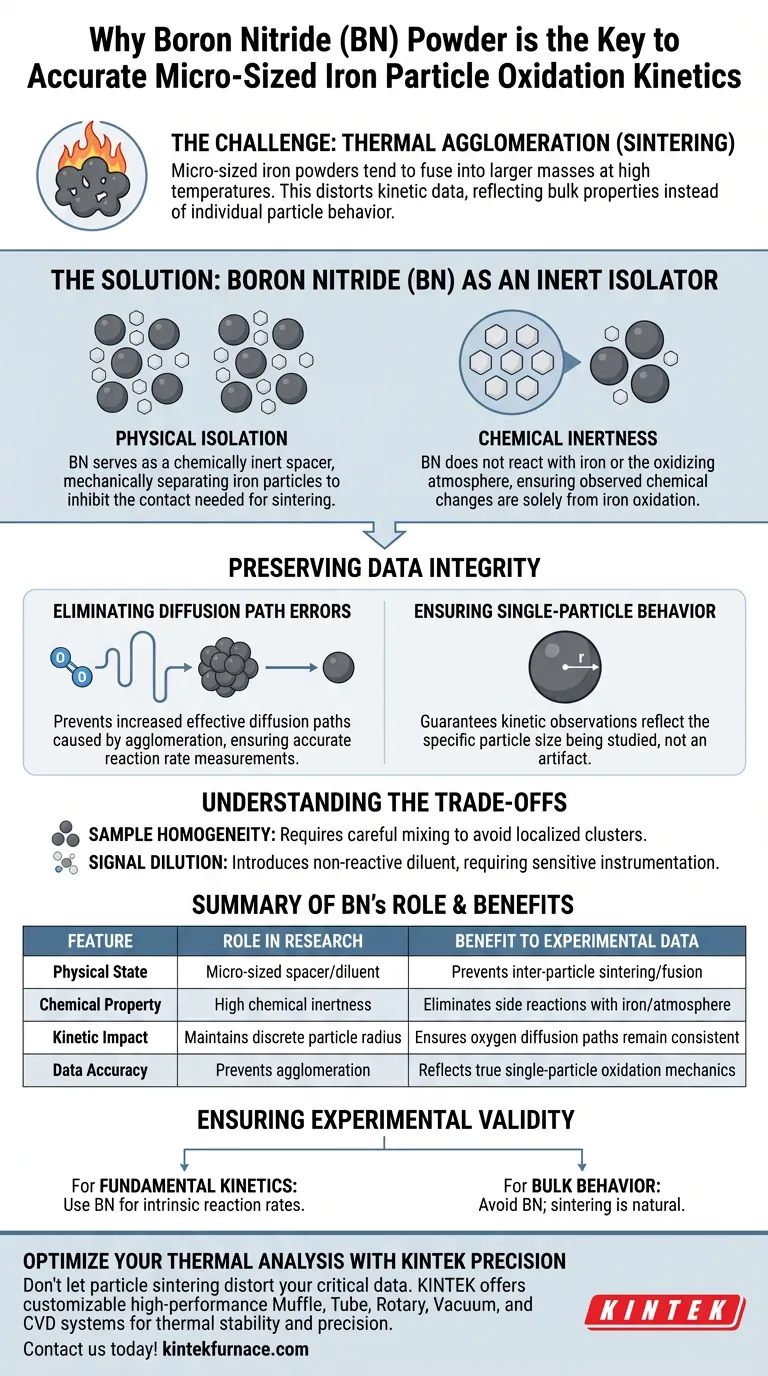
Boron Nitride (BN) powder serves as a critical, chemically inert spacer used to separate micro-sized iron particles during high-temperature experiments. Its primary function is to physically isolate these particles to prevent them from fusing together, ensuring that the resulting data accurately represents the oxidation behavior of individual particles rather than a fused mass.
By preventing inter-particle sintering, Boron Nitride eliminates distortions in kinetic data caused by particle agglomeration. This ensures experimental results reflect true single-particle oxidation mechanics rather than the slower diffusion rates of a bulk volume.
The Challenge: Thermal Agglomeration
Inter-Particle Sintering
When micro-sized iron powders are subjected to the high temperatures required for oxidation studies, the individual particles naturally tend to bond together.
This process, known as sintering, causes distinct particles to fuse into larger, irregular volumes.
The Distortion of Kinetic Data
If sintering occurs, the experimental data no longer represents the behavior of micro-sized particles.
Instead, the results reflect the properties of a bulkier, agglomerated mass, which fundamentally skews the oxidation kinetics you are trying to measure.
The Solution: Boron Nitride as an Isolator
Physical Isolation
Boron Nitride is introduced as a diluent to create space between the iron particles.
By mechanically separating the iron, BN effectively inhibits the contact necessary for sintering to occur, maintaining the discrete nature of the iron powder throughout the heating process.
Chemical Inertness
Crucially, Boron Nitride is chemically inert in this context.
It does not react with the iron or the oxidizing atmosphere, ensuring that the observed chemical changes are strictly due to iron oxidation and not a side reaction with the diluent.
Preserving Data Integrity
Eliminating Diffusion Path Errors
When particles agglomerate, the distance oxygen must travel to react with the metal increases significantly.
This creates increased effective diffusion paths, which slows down the reaction rate artificially.
Ensuring Single-Particle Behavior
By using BN to prevent this agglomeration, researchers ensure the oxygen diffusion path remains consistent with the radius of a single particle.
This guarantees that the kinetic observations are accurate to the specific particle size being studied, rather than an artifact of the experimental setup.
Understanding the Trade-offs
Sample Homogeneity
While BN is excellent for isolation, its use requires careful mixing.
If the diluent is not distributed evenly, localized clusters of iron may still sinter, leading to inconsistent data within the same sample batch.
Signal Dilution
Introducing a non-reactive diluent naturally reduces the density of the reactive material (iron) in the sample holder.
This means the total oxidative signal (such as mass gain) will be lower relative to the total volume, requiring sensitive instrumentation to measure the kinetics accurately.
Ensuring Experimental Validity
If your primary focus is fundamental kinetics:
- Use Boron Nitride to isolate particles, ensuring your data models intrinsic reaction rates rather than bulk diffusion limitations.
If your primary focus is bulk material behavior:
- Avoid using a diluent like BN, as sintering and agglomeration are natural parts of how bulk powders behave under heat.
Boron Nitride provides the physical separation necessary to view the chemistry of iron oxidation in its purest, particle-level form.
Summary Table:
| Feature | Role of Boron Nitride (BN) in Research | Benefit to Experimental Data |
|---|---|---|
| Physical State | Acts as a micro-sized spacer/diluent | Prevents inter-particle sintering and fusion |
| Chemical Property | High chemical inertness | Eliminates side reactions with iron or atmosphere |
| Kinetic Impact | Maintains discrete particle radius | Ensures oxygen diffusion paths remain consistent |
| Data Accuracy | Prevents agglomeration | Reflects true single-particle oxidation mechanics |
Optimize Your Thermal Analysis with KINTEK Precision
Don't let particle sintering distort your critical research data. At KINTEK, we understand the delicate balance of high-temperature experiments. Backed by expert R&D and world-class manufacturing, we provide high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems—all fully customizable to meet your unique laboratory requirements.
Whether you are studying oxidation kinetics or developing new materials, our advanced high-temperature furnaces ensure the thermal stability and precision you need. Contact us today to find the perfect solution for your lab!
Visual Guide
References
- Jonas Spielmann, Ulrike I. Kramm. Exploring the oxidation behavior of undiluted and diluted iron particles for energy storage: Mössbauer spectroscopic analysis and kinetic modeling. DOI: 10.1039/d3cp03484d
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Chairside Dental Porcelain Zirconia Sintering Furnace with Transformer for Ceramic Restorations
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- Vacuum Heat Treat Sintering and Brazing Furnace
People Also Ask
- What is the significance of the vacuum oven drying process in the preparation of MnO@WAC electrode sheets? Expert Guide
- How does a high-precision temperature control system influence the nanoparticle size? Master Catalyst Activation
- Why is a laboratory oven used for constant temperature treatment of celadon? Ensure Peak Measurement Accuracy
- Why is a nitrogen protection system necessary for LPF resin synthesis? Ensure Purity in Lab Polymerization
- What is the purpose of analyzing dust from furnace walls using XRD? Confirm Magnesium Evaporation in AM60 Alloy
- What is the function of an electric arc furnace in the preparation of aluminum-silicon model alloys? Expert Insights
- Why must (MnFeNiCo)3O4 materials undergo a secondary calcination? Key Steps to Optimizing FCC Spinel Structure
- How does a high-precision temperature control system affect high-entropy materials? Unlock Material Performance






