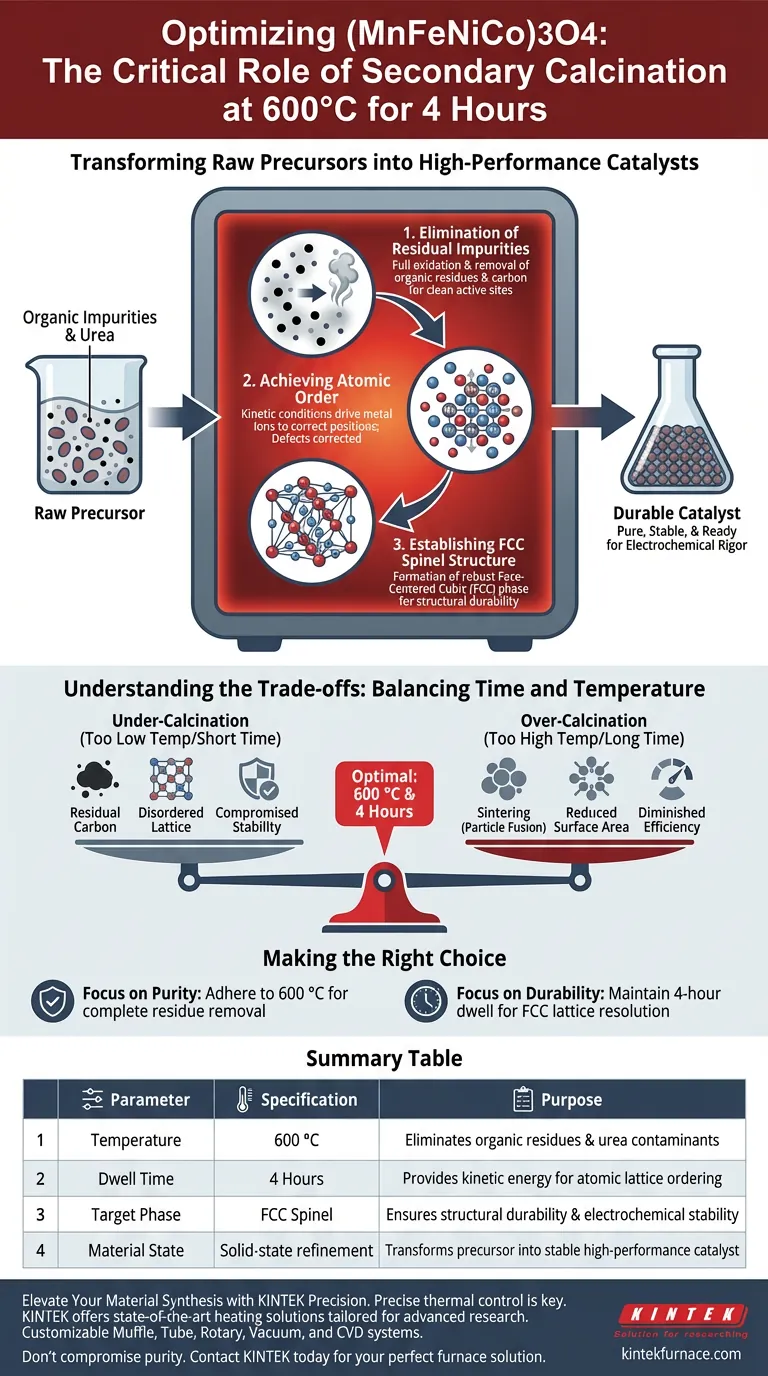
Secondary calcination serves as the critical finalization step for synthesizing high-performance (MnFeNiCo)3O4 materials. This specific thermal treatment—conducted at 600 °C for 4 hours—is required to strip away remaining organic impurities from the initial combustion and to force the atomic structure into a thermodynamically stable configuration. Without this step, the material would lack the purity and crystalline order necessary for effective application.
The process transforms a raw precursor into a durable catalyst by driving the formation of a pure, face-centered cubic (FCC) spinel structure. By eliminating combustion residues and enabling complete lattice ordering, this thermal treatment ensures the material can withstand the rigors of electrocatalytic processes.

The Mechanics of Material Refinement
Elimination of Residual Impurities
The initial combustion reaction used to create the precursor material is rarely 100% efficient. It often leaves behind organic residues, specifically unreacted urea or carbon.
If left in the material, these residues can block active sites or interfere with surface reactions. The high-temperature environment of the furnace ensures these contaminants are fully oxidized and removed.
Achieving Atomic Order
Creating a complex multi-metal oxide like (MnFeNiCo)3O4 requires precise atomic arrangement. The secondary calcination provides the necessary kinetic conditions to mobilize the atoms within the solid.
This thermal energy allows the metal ions to migrate to their correct positions within the crystal lattice. This process, known as lattice ordering, corrects defects that formed during the rapid initial synthesis.
Establishing the FCC Spinel Structure
The ultimate goal of this heat treatment is phase purity. The 600 °C setpoint is tuned to favor the formation of a stable face-centered cubic (FCC) spinel structure.
This specific crystalline phase is known for its robustness. By locking the atoms into this configuration, the material gains significant structural durability, preventing it from degrading during harsh electrochemical reactions.
Understanding the Trade-offs
The Balance of Time and Temperature
While 600 °C is the target for this specific material, deviating from this parameter presents risks.
Insufficient temperature or duration will result in an "under-cooked" material. This leads to residual carbon contamination and a disordered lattice, which compromises catalytic activity and stability.
The Risk of Over-Calcination
Conversely, exceeding the necessary temperature or duration can lead to sintering.
If the material is heated too aggressively, the particles may fuse together. This reduces the active surface area, diminishing the material's efficiency despite its high purity.
Making the Right Choice for Your Synthesis
To ensure you achieve a catalyst that is both pure and mechanically robust, consider your specific performance targets:
- If your primary focus is maximum chemical purity: Strictly adhere to the 600 °C temperature floor to ensure the complete oxidation and removal of stubborn organic residues like unreacted urea.
- If your primary focus is long-term structural durability: Do not shorten the 4-hour dwell time, as this duration provides the necessary kinetic window for the lattice to fully resolve into the stable FCC spinel phase.
The precision of your thermal treatment defines the difference between a volatile precursor and a reliable, high-performance catalyst.
Summary Table:
| Parameter | Specification | Purpose |
|---|---|---|
| Temperature | 600 °C | Eliminates organic residues & urea contaminants |
| Dwell Time | 4 Hours | Provides kinetic energy for atomic lattice ordering |
| Target Phase | FCC Spinel | Ensures structural durability & electrochemical stability |
| Material State | Solid-state refinement | Transforms precursor into stable high-performance catalyst |
Elevate Your Material Synthesis with KINTEK Precision
Precise thermal control is the difference between a failed precursor and a high-performance catalyst. KINTEK provides state-of-the-art heating solutions tailored for advanced material research. Backed by expert R&D and manufacturing, we offer Muffle, Tube, Rotary, Vacuum, and CVD systems, all fully customizable to meet the rigorous 600 °C demands of your (MnFeNiCo)3O4 calcination protocols.
Don't let temperature fluctuations compromise your FCC spinel purity. Contact KINTEK today to find the perfect high-temperature furnace for your laboratory’s unique needs.
Visual Guide
References
- Milad Zehtab Salmasi, Hua Song. Tuning High-Entropy Oxides for Oxygen Evolution Reaction Through Electrocatalytic Water Splitting: Effects of (MnFeNiCoX)3O4 (X = Cr, Cu, Zn, and Cd) on Electrocatalytic Performance. DOI: 10.3390/catal15090827
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
People Also Ask
- What is the function of a ball mill in the raw material pretreatment stage for the szaibelyite vacuum thermal reduction process?
- How does a stable constant temperature environment influence the structural development of LDHs during aging?
- How does the QIO algorithm improve temperature control precision in electric furnaces? Achieve Global Optimization
- Why is a vacuum storage environment necessary for solid polymer electrolyte films? Ensure Film Integrity & Data Accuracy
- What is the primary function of a constant temperature drying oven in ceramic powder pretreatment? Get Expert Results
- What role does a precision mass loss measurement system play? Identifying Vapor Pressure in High-Temp Furnaces
- What is the purpose of mixing aluminum and iron powders at a specific atomic ratio? Optimize Al-Fe Alloy Phases
- What is the objective of GC-MS analysis on bio-oil? Unlock Chemical Value and Industrial Utility



















