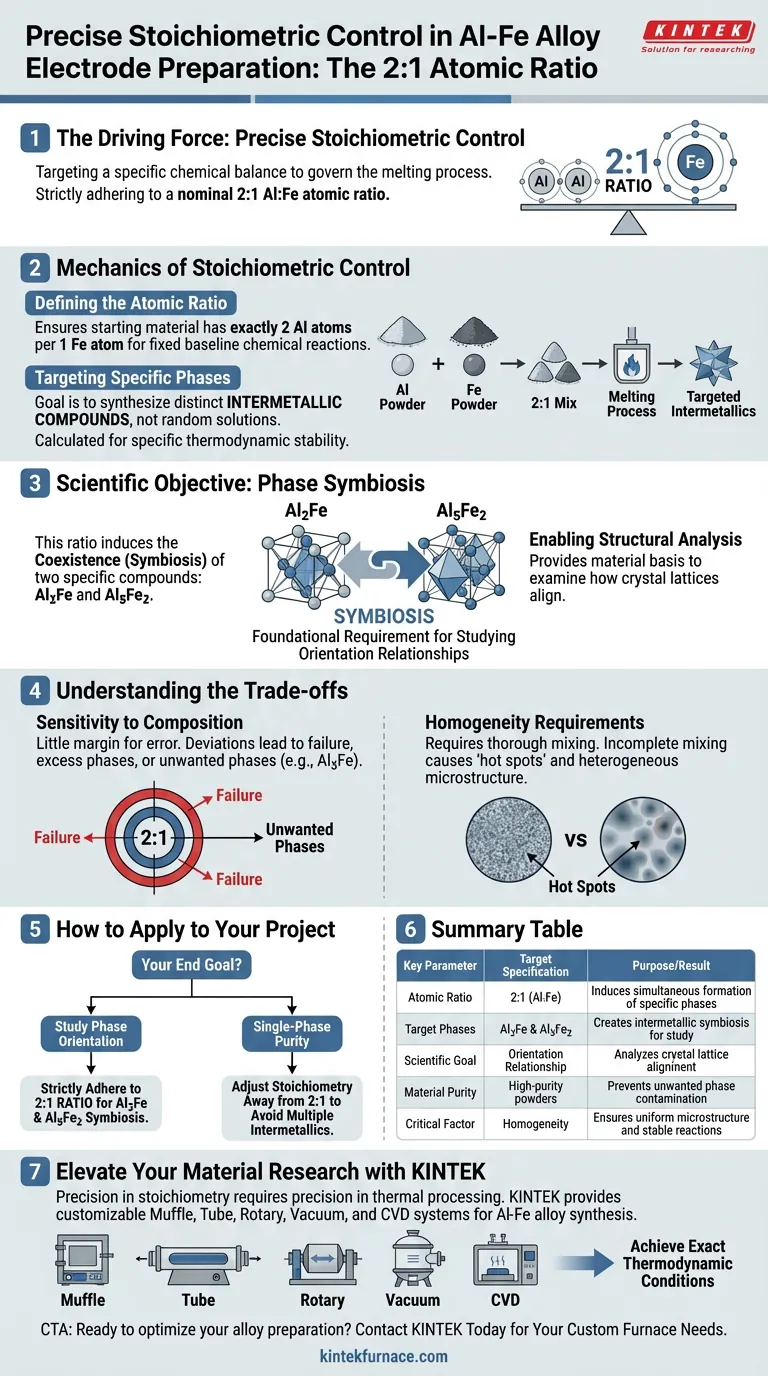
Precise stoichiometric control is the driving force behind mixing aluminum and iron powders at a specific atomic ratio during electrode preparation. By strictly adhering to a nominal 2:1 atomic ratio, researchers can intentionally target a specific chemical balance that governs the melting process. This precision is required to induce the simultaneous formation and coexistence of two distinct phases within the final alloy.
By locking the mixture at a 2:1 ratio, you create the necessary conditions for the symbiosis of Al2Fe and Al5Fe2 intermetallic compounds. This specific phase coexistence is the foundational requirement for studying the orientation relationships between these two structures.

The Mechanics of Stoichiometric Control
Defining the Atomic Ratio
In materials science, the properties of an alloy are dictated by the proportion of its constituent atoms. For Al-Fe alloys, a random mixture will result in unpredictable material characteristics.
Using a high-purity nominal 2:1 ratio ensures the starting material has exactly two aluminum atoms for every iron atom. This provides a fixed baseline for the chemical reactions that occur during melting.
Targeting Specific Phases
The goal of this specific ratio is not to create a random solid solution, but to synthesize specific intermetallic compounds.
These compounds are distinct chemical species with defined crystal structures. The 2:1 input is calculated to drive the system toward specific thermodynamic stability points.
The Scientific Objective: Phase Symbiosis
Inducing Coexistence
The primary reference indicates that this specific ratio causes the symbiosis of two specific compounds: Al2Fe and Al5Fe2.
Rather than yielding a single uniform phase, the 2:1 ratio forces these two distinct intermetallics to form together. This "symbiotic" presence is likely due to the specific saturation points of iron in the aluminum matrix at this ratio.
Enabling Structural Analysis
The ultimate purpose of creating this mixture is research-oriented.
By ensuring both Al2Fe and Al5Fe2 are present in the solidified alloy, scientists provide the material basis required to study their interactions. Specifically, it allows for the detailed examination of orientation relationships—how the crystal lattice of one phase aligns with the other.
Understanding the Trade-offs
Sensitivity to Composition
It is critical to understand that this process allows for very little margin of error.
If the atomic ratio deviates significantly from 2:1, the resulting alloy may fail to produce the required symbiosis. You may end up with an excess of one phase or the introduction of unwanted phases (such as Al3Fe), rendering the orientation study impossible.
Homogeneity Requirements
Achieving this ratio requires more than just weighing powders; the mixing must be thorough.
Incomplete mixing can lead to localized "hot spots" of aluminum or iron. This results in a heterogeneous microstructure where the target Al2Fe and Al5Fe2 phases do not interact as intended.
How to Apply This to Your Project
If you are preparing Al-Fe alloy electrodes, your mixing strategy depends entirely on your end goal.
- If your primary focus is studying phase orientation: Adhere strictly to the 2:1 atomic ratio to force the coexistence of Al2Fe and Al5Fe2.
- If your primary focus is single-phase purity: You must adjust the stoichiometry away from the 2:1 ratio to avoid the symbiotic formation of multiple intermetallics.
Success in this preparation relies on viewing the powder ratio not as a rough guideline, but as a precise coordinate for microstructural engineering.
Summary Table:
| Key Parameter | Target Specification | Purpose/Result |
|---|---|---|
| Atomic Ratio | 2:1 (Al:Fe) | Induces simultaneous formation of specific phases |
| Target Phases | Al2Fe & Al5Fe2 | Creates intermetallic symbiosis for study |
| Scientific Goal | Orientation Relationship | Analyzes crystal lattice alignment between phases |
| Material Purity | High-purity powders | Prevents unwanted phase contamination (e.g., Al3Fe) |
| Critical Factor | Homogeneity | Ensures uniform microstructure and stable reactions |
Elevate Your Material Research with KINTEK
Precision in stoichiometry requires precision in thermal processing. KINTEK provides industry-leading, customizable Muffle, Tube, Rotary, Vacuum, and CVD systems designed to meet the rigorous demands of Al-Fe alloy synthesis and intermetallic research. Our expert R&D and manufacturing capabilities ensure your lab achieves the exact thermodynamic conditions needed for perfect phase symbiosis.
Ready to optimize your alloy preparation? Contact KINTEK Today to Discuss Your Custom Furnace Needs
Visual Guide
References
- Yibo Liu, Lifeng Zhang. Orientation Relationship of Intergrowth Al2Fe and Al5Fe2 Intermetallics Determined by Single-Crystal X-ray Diffraction. DOI: 10.3390/met14030337
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- Chairside Dental Porcelain Zirconia Sintering Furnace with Transformer for Ceramic Restorations
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- Custom Made Versatile CVD Tube Furnace Chemical Vapor Deposition CVD Equipment Machine
- Multi Zone Laboratory Quartz Tube Furnace Tubular Furnace
People Also Ask
- What is the function of a laboratory vacuum drying oven in the preparation of 6FDA-TFDB dense membranes?
- How does a high-precision PID temperature controller ensure the quality of biochar? Master Teff Husk Pyrolysis
- What is the purpose of using a thermal evaporation coating system? Enhancing I-V Testing Accuracy for Nanocomposites
- Why are acid washing and vacuum drying ovens required after carbon activation? Unlock Maximum Purity and Pore Access
- What is the primary purpose of sealing the reaction bottle at 80°C for RMF synthesis? Ensure Optimal Polycondensation
- What is the specific function of a high-temperature laboratory furnace during the activation of kaolin-based catalysts?
- What morphological changes occur in POMOF after treatment? Unlock High Catalytic Performance via Thermal Evolution
- Why are graphite molds preheated to 800 °C for Invar 36 casting? Unlock High-Quality Ingot Production



















