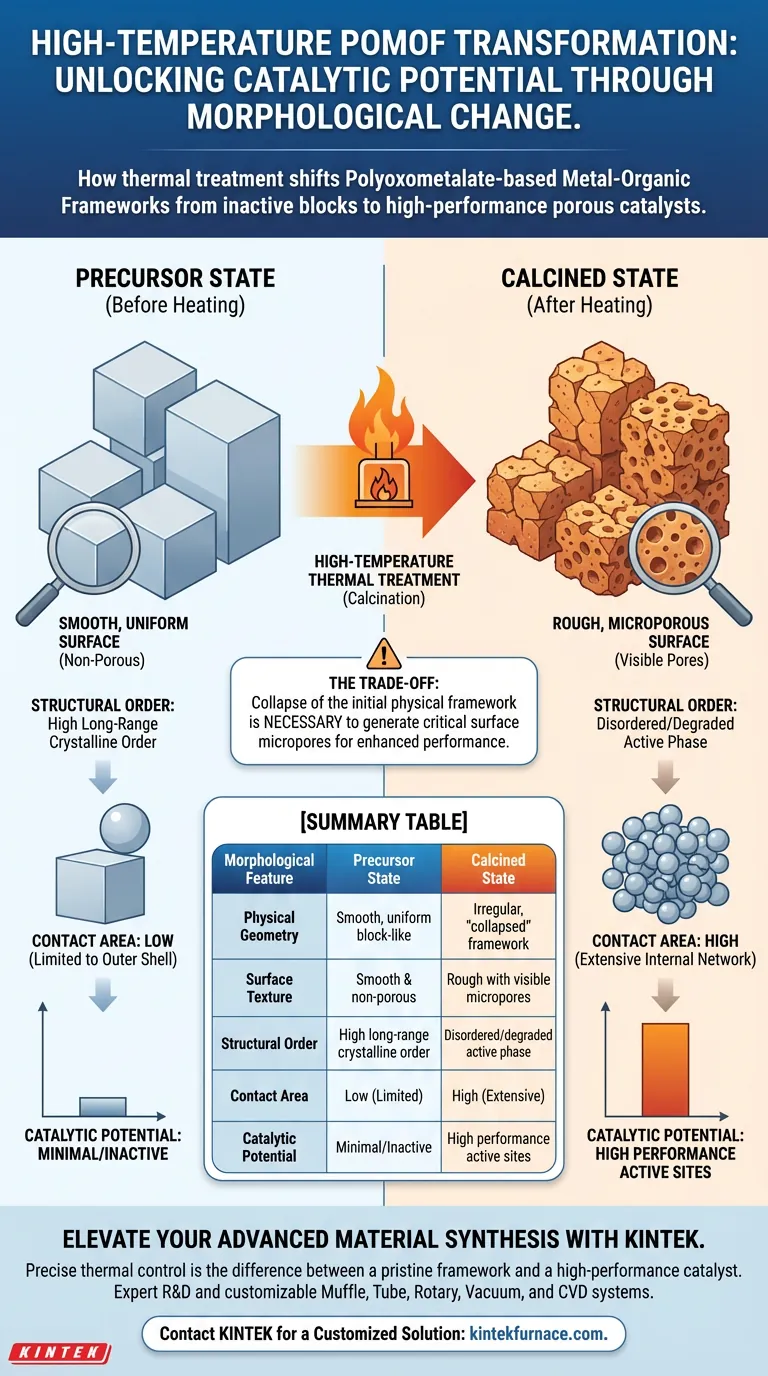
High-temperature thermal treatment fundamentally transforms the micro-morphology of Polyoxometalate-based Metal-Organic Framework (POMOF) materials. What begins as a solid, uniform structure undergoes a distinct physical evolution, transitioning from smooth blocks into a rougher, "collapsed" state characterized by the emergence of numerous surface micropores.
The calcination process involves a strategic trade-off: the collapse of the initial physical framework is necessary to generate critical surface micropores, which significantly increase the effective contact area and enhance catalytic performance.

The Evolution of Micro-Morphology
The Precursor State
Before thermal treatment, POMOF precursors typically exhibit a distinct geometry. They appear as smooth, block-like structures.
At this stage, the surface is relatively uniform. The material lacks the visible texture and porosity that define its activated state.
The Phenomenon of Structural Collapse
When subjected to a high-temperature furnace, the material does not simply harden; it degrades physically. The heat causes the original, organized framework to break down.
This process is referred to as structural collapse. The smooth exterior gives way to a more irregular, degraded form as the internal components react to the thermal stress.
Formation of Micropores
Simultaneous with this collapse is the creation of new features. The treatment leads to the formation of visible micropores across the surface of the material.
These pores replace the smooth finish of the precursor. They represent voids opened up during the decomposition and restructuring of the organic and inorganic components.
Functional Implications of the Change
Increasing Effective Contact Area
The primary benefit of this morphological change is geometric. By transitioning from a smooth block to a porous, roughened structure, the material’s effective contact area increases dramatically.
Where the precursor limited interaction to its outer shell, the calcined material offers a vast network of surfaces for chemical interaction.
Enhancing Catalytic Performance
This increase in surface area is directly linked to utility. The newly formed micropores provide accessible sites for reactants to interact with the material.
Consequently, the overall catalytic performance of the POMOF is improved. The "collapsed" structure is, counter-intuitively, more chemically active than the pristine, smooth precursor.
Understanding the Trade-offs
Loss of Long-Range Order
It is important to recognize that "structural collapse" implies the destruction of the original MOF crystal lattice.
You are effectively trading the highly ordered, crystalline nature of the precursor for a more disordered, but functionally porous, active phase.
Reliance on Thermal Precision
The generation of micropores is a result of decomposition. This implies that the temperature profile must be controlled carefully.
Insufficient heat may leave the smooth blocks intact and non-porous, while excessive heat could potentially lead to complete sintering or loss of active sites, rather than the desired microporous collapse.
Implications for Material Design
To maximize the utility of POMOF materials, you must align the processing stage with your end goal.
- If your primary focus is high catalytic activity: Ensure the material undergoes sufficient calcination to trigger structural collapse and maximize the formation of surface micropores.
- If your primary focus is analyzing the pristine framework: You must examine the material in its precursor stage, characterized by smooth, block-like structures, before thermal degradation occurs.
The destruction of the initial form is the essential step in unlocking the material's potential as a high-performance catalyst.
Summary Table:
| Morphological Feature | Precursor State (Before Heating) | Calcined State (After Heating) |
|---|---|---|
| Physical Geometry | Smooth, uniform block-like structures | Irregular, "collapsed" framework |
| Surface Texture | Smooth and non-porous | Rough with visible micropores |
| Structural Order | High long-range crystalline order | Disordered/degraded active phase |
| Contact Area | Low (limited to outer shell) | High (extensive internal network) |
| Catalytic Potential | Minimal/Inactive | High performance active sites |
Elevate Your Advanced Material Synthesis with KINTEK
Precise thermal control is the difference between a pristine framework and a high-performance catalyst. Backed by expert R&D and manufacturing, KINTEK offers a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems, all fully customizable to meet the rigorous temperature profiles required for POMOF transformation.
Whether you are scaling up production or conducting delicate lab-scale research, our high-temperature furnaces provide the uniformity and precision needed to optimize surface micropore formation.
Ready to refine your thermal processing? Contact KINTEK today for a customized solution!
Visual Guide
References
- Zi‐Qing Liu, Bao‐Li Fei. Mixed Metal Oxide Derived from Polyoxometalate-Based Metal–Organic Framework as a Bi-Functional Heterogeneous Catalyst for Wastewater Treatment. DOI: 10.3390/catal15010076
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1400℃ Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- Why is an industrial drying oven necessary for Boron Carbide mixed slurries? Ensure Coating Integrity & Precision
- Why does the use of a forced-air drying oven often lead to increased particle size? Avoid Silica Agglomeration
- What is made in a dental lab? Discover the Custom Prosthetics for Your Smile
- How do CVT and hPLD process conditions for Nb1+xSe2 crystals differ? Exploring Equilibrium vs. Dynamic Growth
- Why is a constant temperature blast drying oven necessary for biomass carbon impregnation? Optimize Material Structure
- Why is it necessary to use an annealing furnace at 350°C for three hours? Ensuring Glass Stability and Clarity
- What is the importance of transferring freshly deposited CuO films directly into a 125°C oven? Ensure Film Adhesion
- How does the extended isothermal calcination in a furnace contribute to crystalline quality? Boost Material Purity



















