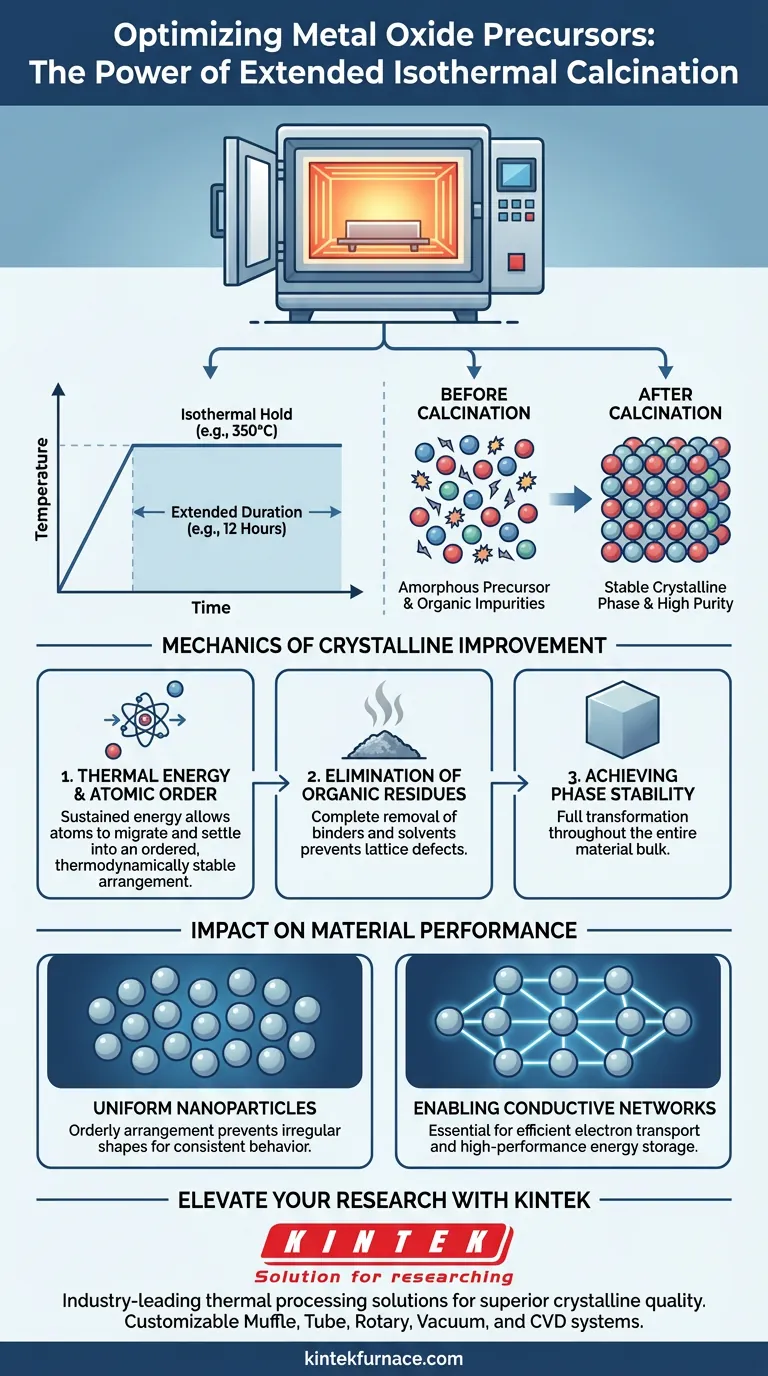
Extended isothermal calcination acts as a comprehensive stabilization process for metal oxide precursors, fundamentally altering their internal structure. By holding a constant temperature (e.g., 350°C) for a prolonged period (e.g., 12 hours), the furnace provides the sustained thermal energy required to force atoms into an orderly lattice arrangement while simultaneously driving off organic impurities.
The primary function of extended calcination is to drive atomic organization. It ensures the complete transformation of raw precursors into a stable, uniform crystalline phase, which is a prerequisite for high-performance applications like energy storage.

The Mechanics of Crystalline Improvement
To understand why extended time is necessary, we must look at what is happening at the atomic level during the hold phase.
Thermal Energy and Atomic Order
Crystallization is not instantaneous; it requires energy and time. The extended hold at temperatures like 350°C provides the thermal energy necessary for atoms to migrate from a chaotic state to a structured one.
This sustained energy input allows the atoms to settle into a thermodynamically stable, orderly arrangement. Without this duration, the material might remain amorphous or poorly crystallized.
Elimination of Organic Residues
Precursors often contain organic binders or residual solvents from the synthesis process. Extended calcination ensures the complete removal of these organic residues.
If these residues are not fully burned off, they act as impurities that interrupt the crystal lattice. A 12-hour hold ensures that the final material is chemically pure.
Achieving Phase Stability
The goal of calcination is to reach a specific, stable crystalline phase. The prolonged exposure ensures that the transformation is complete throughout the entire bulk of the material, rather than just on the surface.
Impact on Material Performance
The structural changes driven by calcination have direct consequences on how the material performs in real-world applications.
Creating Uniform Nanoparticles
In the context of materials like Cobalt Oxide, extended isothermal calcination results in highly uniform nanoparticles.
The orderly arrangement of atoms prevents the formation of irregular shapes or sizes. This morphological uniformity is critical for consistent material behavior.
Enabling Conductive Networks
For energy storage devices, the physical connection between particles is vital. The uniform nanoparticles produced by this process are essential for building effective conductive networks.
High-quality crystallinity ensures efficient electron transport, directly impacting the efficiency and capacity of the final energy storage device.
The Critical Balance of Time and Quality
While the benefits are clear, it is important to understand the operational constraints of this process.
The Necessity of Duration
The process is inherently time-consuming. A 12-hour hold is a significant investment in processing time that limits throughput speed.
The Risk of Rushing
Attempting to shorten this window introduces the risk of incomplete transformation. Insufficient time may leave organic residues trapped inside the material or result in a disordered atomic structure, compromising the conductive network.
Making the Right Choice for Your Goal
When designing a synthesis protocol for metal oxides, consider how the calcination parameters align with your performance requirements.
- If your primary focus is Phase Purity: Ensure the hold time is sufficient to fully burn off all organic residues to prevent lattice defects.
- If your primary focus is Device Performance: Prioritize extended isothermal holds to achieve the particle uniformity required for robust conductive networks.
Extended calcination is the bridge between a raw chemical precursor and a high-performance functional material.
Summary Table:
| Process Element | Impact on Crystalline Quality | Application Benefit |
|---|---|---|
| Sustained Thermal Energy | Facilitates atomic migration to orderly lattice | High thermodynamical stability |
| Extended Duration | Ensures complete removal of organic residues | High chemical purity & fewer defects |
| Phase Uniformity | Transformation occurs through entire material bulk | Consistent material behavior |
| Particle Morphology | Produces highly uniform nanoparticles | Enhanced conductive networks |
Elevate Your Material Research with KINTEK
High-performance energy storage and advanced material synthesis demand absolute precision in thermal processing. KINTEK provides the industry-leading solutions required to achieve superior crystalline quality. Backed by expert R&D and manufacturing, we offer a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems, all fully customizable to meet your specific isothermal calcination needs.
Don't let incomplete transformations compromise your device performance. Contact us today to discover how our specialized lab high-temperature furnaces can provide the thermal stability and uniformity your research deserves.
Visual Guide
References
- Changwei Shan, Liwei Mi. Co<sub>1−<i>x</i></sub>S@CNT composite with a three-dimensional skeleton for high-performance magnesium–lithium hybrid batteries. DOI: 10.1039/d3ma01089a
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 600T Vacuum Induction Hot Press Vacuum Heat Treat and Sintering Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
People Also Ask
- How do lab high-temp furnaces and air quenching coordinate in o-LISO synthesis? Master the Thermal Transition
- What is the purpose of sintering furnaces? Transform Powders into Strong, Dense Materials
- Why is a vacuum sealing process necessary for the synthesis of TaAs2 single crystals? Ensuring Purity in CVT Method
- Why is thermal treatment of Mn1/CeO2 catalysts necessary? Unlock Peak Activation and Purity
- Why is a constant temperature drying oven utilized at 40 °C for clayey raw materials? Ensure Mineral Integrity.
- What is the role of an industrial high-speed ball mill in kaolin pretreatment? Enhance Reactivity & Surface Area
- What is the significance of maintaining an inert nitrogen atmosphere during molten salt activation? Ensure Pore Purity
- What is zirconium dioxide and how is it stabilized for dental use? Discover the Science Behind Durable Dental Ceramics



















