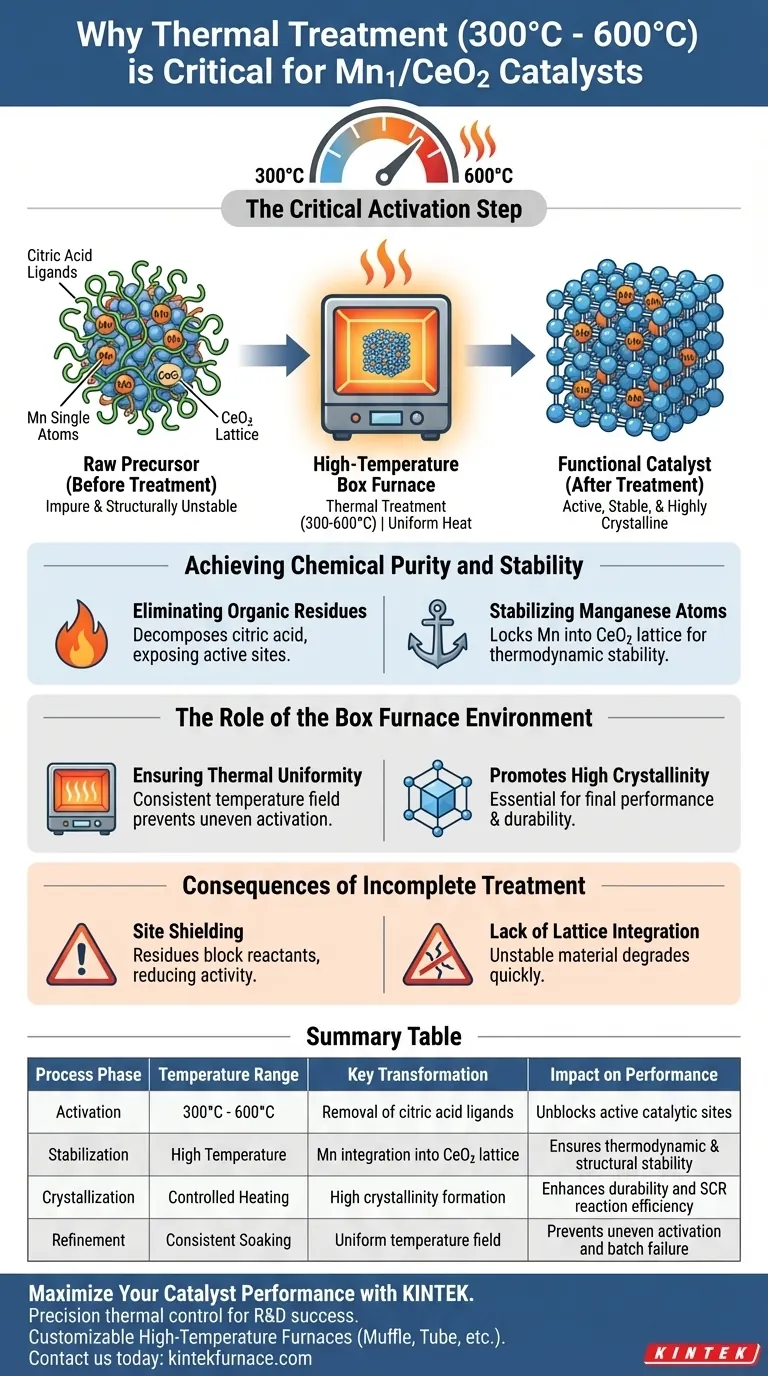
Thermal treatment between 300 °C and 600 °C is the critical activation step for Mn1/CeO2 catalysts. This process is required to strip away organic residues like citric acid and force the manganese single atoms to stabilize thermodynamically within the cerium dioxide lattice. Without this specific heating regime, the catalyst remains impure and structurally unstable.
This thermal processing stage bridges the gap between a raw precursor and a functional catalyst. It eliminates site-blocking impurities and ensures a uniform, highly crystalline structure, directly enabling efficient NH3-SCR reaction activity.

Achieving Chemical Purity and Stability
The primary function of this thermal treatment is to transition the material from a chemical mixture to an active catalyst.
Eliminating Organic Residues
The precursor material initially contains citric acid ligands and other impurities derived from the synthesis process.
If left untreated, these residues physically shield the active catalytic sites. High-temperature treatment decomposes these organics, exposing the surface for reaction.
Stabilizing Manganese Atoms
Mere mixing is insufficient for single-atom catalysis; the atoms must be anchored correctly.
The heat provides the energy necessary for thermodynamic stabilization. This locks the manganese single atoms into the cerium dioxide lattice, ensuring they remain robust during operation.
The Role of the Box Furnace Environment
The choice of equipment is as critical as the temperature itself. The high-temperature box furnace offers specific conditions that standard heating methods may lack.
Ensuring Thermal Uniformity
A box furnace generates a uniform temperature field around the sample.
This consistency ensures that every part of the catalyst batch undergoes the same transformation, preventing uneven activation.
Promoting High Crystallinity
The stable thermal environment facilitates the transformation of the material into a highly crystalline state.
This crystalline structure is essential for the catalyst's final performance and durability in NH3-SCR reactions.
The Consequences of Incomplete Treatment
Understanding why this step is strictly necessary requires looking at the failure modes of improper treatment.
The Problem of Site Shielding
If the temperature is too low or the time too short, precursor residues remain on the surface.
These residues act as contaminants, blocking the interaction between the catalyst and the reactants, thereby drastically reducing activity.
Lack of Lattice Integration
Without sufficient thermal energy, the manganese atoms may not fully integrate into the lattice.
This results in a thermodynamically unstable material that may degrade quickly under reaction conditions.
Making the Right Choice for Your Goal
When designing your synthesis protocol, ensure your thermal treatment parameters align with the specific needs of the Mn1/CeO2 system.
- If your primary focus is Maximum Activity: Prioritize the upper end of the temperature range to ensure the total removal of all citric acid ligands that shield active sites.
- If your primary focus is Structural Stability: Ensure the box furnace provides a perfectly uniform field to guarantee the thermodynamic integration of manganese into the lattice.
Precise thermal control in this specific environment is the difference between a blocked precursor and a high-performance catalyst.
Summary Table:
| Process Phase | Temperature Range | Key Transformation | Impact on Performance |
|---|---|---|---|
| Activation | 300°C - 600°C | Removal of citric acid ligands | Unblocks active catalytic sites |
| Stabilization | High Temperature | Mn integration into CeO2 lattice | Ensures thermodynamic & structural stability |
| Crystallization | Controlled Heating | High crystallinity formation | Enhances durability and SCR reaction efficiency |
| Refinement | Consistent Soaking | Uniform temperature field | Prevents uneven activation and batch failure |
Maximize Your Catalyst Performance with KINTEK
Precision is the difference between a raw precursor and a high-performance Mn1/CeO2 catalyst. At KINTEK, we understand that achieving a perfectly uniform temperature field and precise thermal control is essential for your R&D success.
Backed by expert R&D and manufacturing, KINTEK offers state-of-the-art Muffle, Tube, Rotary, Vacuum, and CVD systems, along with other lab high-temperature furnaces—all fully customizable to meet your unique synthesis needs. Whether you are focusing on maximizing activity or ensuring structural stability, our equipment provides the reliability you require.
Ready to elevate your material synthesis? Contact us today to find the perfect high-temperature furnace for your lab!
Visual Guide
References
- Weibin Chen, Ruqiang Zou. Designer topological-single-atom catalysts with site-specific selectivity. DOI: 10.1038/s41467-025-55838-6
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
People Also Ask
- Why is the adsorption of dioxins more effective using carbon nanotubes (CNTs)? 3x Superior Efficiency Explained
- What is the purpose of high-temperature homogenization annealing? Optimizing Ti-5Al-2.5Sn-0.2C Alloy Ductility
- What is the function of a stainless steel high-pressure reactor in HTC? Optimize Ion-Exchange Resin Conversion
- What is the technical objective of performing thermal oxidation at 625 °C? Mastering SiOx Tunnel Oxide Precision
- Why is a forced-air drying oven necessary for impregnated kaolin catalysts? Achieve Uniform Component Immobilization
- What is the specific purpose of using a laboratory oven for the treatment of copper oxide precipitates? Expert Insights
- What are the primary functions of high-purity nitrogen flow in carbon pyrolysis? Optimize Purity and Pore Structure
- What role does the high-temperature boiling step play in rice husk silica conversion? Boost Your Extraction Yields



















