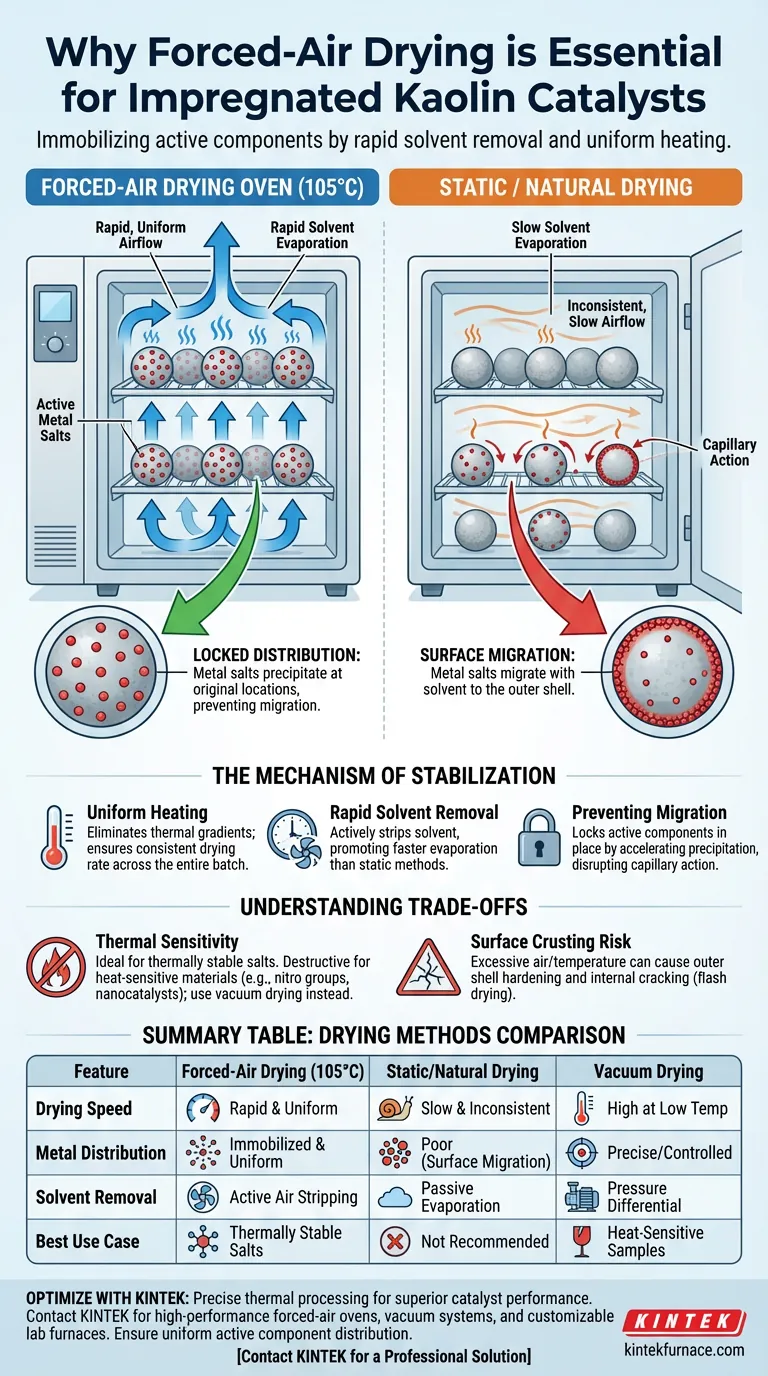
The primary necessity of a forced-air drying oven is to immobilize active components. By utilizing forced hot air circulation at approximately 105°C, the oven ensures rapid solvent removal and uniform heating across the impregnated kaolin. This mechanical action is strictly required to prevent the dissolved metal salts from migrating or redistributing, which inevitably occurs during slower, natural drying methods.
Core Takeaway The success of an impregnated catalyst relies on where the active metals sit on the support. Forced-air drying is a method of "locking" these components in their initial state, preventing capillary forces from moving them around before they solidify.

The Mechanism of Stabilization
Uniform Heating Through Circulation
In static drying environments, heat distribution can be inconsistent, leading to "hot spots" and "cold spots."
Forced-air circulation eliminates these thermal gradients. It ensures that every particle of the kaolin carrier experiences the same temperature simultaneously, resulting in a consistent drying rate across the entire batch.
Rapid Solvent Removal
Speed is a functional requirement, not just a time-saver.
The forced airflow actively strips evaporated solvent away from the catalyst surface. This promotes a faster rate of evaporation than static ovens, which is essential for the immediate precipitation of the active ingredients.
Preventing Component Migration
The Risk of Capillary Action
When a catalyst dries slowly, the solvent moves from the interior of the pore to the outer surface via capillary action.
If the drying is too slow (as in natural drying), the solvent drags the dissolved metal salts along with it. This causes the active components to accumulate on the outer shell of the catalyst rather than remaining evenly distributed throughout the pores.
Locking in Distribution
The forced-air method removes the solvent fast enough to disrupt this migration process.
By accelerating evaporation, the metal salts reach supersaturation and precipitate quickly at their original locations. This preserves the initial distribution state on the carrier surface, ensuring the final catalyst performs predictably.
Understanding the Trade-offs
Thermal Sensitivity Constraints
While forced-air drying at 105°C is ideal for thermally stable metal salts, it is destructive for heat-sensitive materials.
If your catalyst contains organic functional groups (such as nitro groups) or high-activity nanocatalysts prone to oxidation, forced-air drying may cause premature decomposition. In those specific cases, vacuum drying at lower temperatures is the required alternative.
The Risk of Surface Crusting
There is a balance between "rapid drying" and "flash drying."
If the air velocity or temperature is excessively high, the outer surface of the catalyst may dry and harden before the interior. This can trap moisture inside or lead to physical cracking, potentially damaging the pore structure of the kaolin support.
Making the Right Choice for Your Goal
- If your primary focus is uniform metal distribution: Use a forced-air oven to prevent the migration and agglomeration of salts during solvent evaporation.
- If your primary focus is preserving heat-sensitive structures: Avoid forced air; opt for vacuum drying to remove solvents at lower temperatures without oxidation.
- If your primary focus is mechanical integrity: Ensure the drying rate is controlled enough to prevent rapid vaporization from cracking the catalyst tablets.
The forced-air oven is not merely a tool for moisture removal; it is a control device used to freeze the active geometry of the catalyst in place.
Summary Table:
| Feature | Forced-Air Drying (105°C) | Static/Natural Drying | Vacuum Drying |
|---|---|---|---|
| Drying Speed | Rapid & Uniform | Slow & Inconsistent | High at Low Temp |
| Metal Distribution | Immobilized & Uniform | Poor (Surface Migration) | Precise/Controlled |
| Solvent Removal | Active Air Stripping | Passive Evaporation | Pressure Differential |
| Best Use Case | Thermally Stable Salts | Not Recommended | Heat-Sensitive Samples |
Optimize Your Catalyst Production with KINTEK
Precise thermal processing is the key to locking in catalyst performance. Backed by expert R&D and manufacturing, KINTEK offers high-performance forced-air ovens, vacuum systems, and customizable lab furnaces—including Muffle, Tube, and Rotary systems—tailored to your unique research needs. Ensure uniform active component distribution and superior mechanical integrity for your kaolin supports today.
Contact KINTEK for a Professional Solution
Visual Guide
References
- Luqman Buchori, Ndaru Okvitarini. Preparation of KI/KIO3/Methoxide Kaolin Catalyst and Performance Test of Catalysis in Biodiesel Production. DOI: 10.26554/sti.2024.9.2.359-370
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1200℃ Muffle Oven Furnace for Laboratory
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
- Vacuum Dental Porcelain Sintering Furnace for Dental Laboratories
People Also Ask
- What is the purpose of using high-purity nitrogen for nano-zinc oxide experiments? Ensure Data Purity & Accuracy
- How does a benchtop industrial oven improve efficiency? Boost Energy Savings and Space Use
- What are the advantages of ascorbic acid over glucose in LFP synthesis? Achieve Superior Purity and Crystallinity
- Why is it important to choose the right type of heat treatment furnace? Boost Efficiency and Quality in Your Lab or Facility
- What is the purpose of bottom-entry argon injection? Enhance Lithium-ion Battery Safety & Purge Efficiency
- What is the function of a vacuum drying oven in the post-treatment of PPy/alpha-Fe2O3 composite materials? Expert Insights
- How does a high-temperature furnace enhance the availability of phosphorus? Unlock 97.5% Solubility via Calcination
- Why is precise temperature control in a drying oven critical for Li-SPAN battery cycle life? Ensure Peak Performance



















