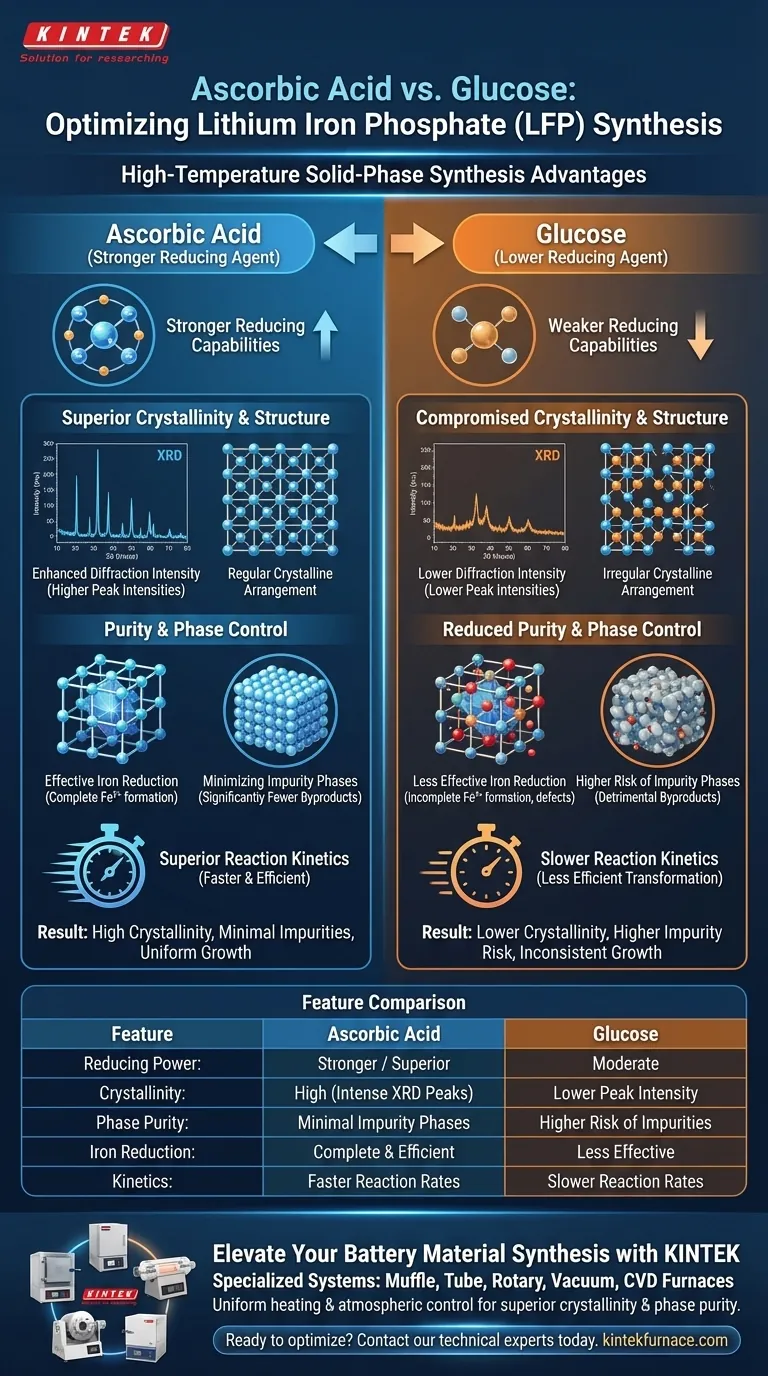
Ascorbic acid outperforms glucose as a reducing agent in the high-temperature solid-phase synthesis of lithium iron phosphate by demonstrating stronger reducing capabilities and superior reaction kinetics. This chemical advantage directly translates to a final product with higher structural regularity and significantly fewer impurity phases.
Ascorbic acid facilitates a more complete and ordered reduction process than glucose. By promoting effective iron reduction, it ensures a highly crystalline structure with minimal impurities, which is fundamental for material stability.

Superior Crystallinity and Structure
Enhanced Diffraction Intensity
When analyzed via X-ray diffraction (XRD), lithium iron phosphate synthesized with ascorbic acid exhibits higher characteristic peak intensities.
This increase in intensity is a direct indicator of superior crystallinity. It suggests that the atomic structure of the material is more perfectly ordered compared to samples synthesized with glucose.
Regular Crystalline Arrangement
The use of ascorbic acid induces a more regular crystalline arrangement within the material.
While glucose functions as a reducing agent, ascorbic acid promotes a more uniform growth mechanism. This regularity is crucial for ensuring consistent electrochemical pathways within the battery material.
Purity and Phase Control
Minimizing Impurity Phases
A critical advantage of ascorbic acid is the production of a material with fewer impurity phases.
Impurity phases are detrimental byproducts that can hinder performance. Ascorbic acid's chemical properties suppress the formation of these unwanted secondary phases more effectively than glucose.
Effective Iron Reduction
The core mechanism behind this purity is the promotion of iron reduction.
Ascorbic acid is more effective at driving the reduction process essential for forming the correct Lithium Iron Phosphate (LFP) phase. This ensures the iron is in the correct oxidation state, preventing the defects often associated with incomplete reduction.
Understanding the Process Variables
Reaction Kinetics
Ascorbic acid offers superior reaction kinetics compared to glucose.
In high-temperature solid-phase synthesis, the speed and efficiency of the reaction determine the homogeneity of the product. Better kinetics imply a more efficient transformation of precursors into the final active material.
The Trade-off of Using Glucose
While glucose is a viable reducing agent, it represents a trade-off in product quality.
The primary reference indicates that relying on glucose results in lower peak intensities and a higher likelihood of impurity phases. Therefore, choosing glucose over ascorbic acid compromises the structural integrity and purity of the final cathode material.
Making the Right Choice for Your Goal
To optimize your solid-phase synthesis process, consider the following based on your specific requirements:
- If your primary focus is maximizing crystallinity: Select ascorbic acid to achieve higher XRD peak intensities and a more regular structural arrangement.
- If your primary focus is phase purity: Prioritize ascorbic acid to ensure effective iron reduction and minimize the formation of detrimental impurity phases.
By leveraging the stronger reducing capabilities of ascorbic acid, you ensure the synthesis of a cleaner, more structurally sound lithium iron phosphate material.
Summary Table:
| Feature | Ascorbic Acid | Glucose |
|---|---|---|
| Reducing Power | Stronger / Superior | Moderate |
| Crystallinity | High (Intense XRD Peaks) | Lower Peak Intensity |
| Phase Purity | Minimal Impurity Phases | Higher Risk of Impurities |
| Iron Reduction | Complete & Efficient | Less Effective |
| Kinetics | Faster Reaction Rates | Slower Reaction Rates |
Elevate Your Battery Material Synthesis with KINTEK
Precision in chemical reduction requires precision in thermal processing. Backed by expert R&D and manufacturing, KINTEK offers specialized Muffle, Tube, Rotary, Vacuum, and CVD systems designed to optimize the high-temperature solid-phase synthesis of lithium iron phosphate.
Whether you are refining reaction kinetics with ascorbic acid or developing next-generation cathode materials, our customizable lab furnaces provide the uniform heating and atmospheric control necessary for superior crystallinity and phase purity.
Ready to optimize your synthesis results? Contact our technical experts today for a tailored high-temperature solution.
Visual Guide
References
- Tengshu Chen, Liyao Chen. Research on the synthesis of lithium iron phosphate using vivianite prepared from municipal sludge. DOI: 10.1038/s41598-025-16378-7
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Spark Plasma Sintering SPS Furnace
- 915MHz MPCVD Diamond Machine Microwave Plasma Chemical Vapor Deposition System Reactor
- Vacuum Heat Treat Sintering Furnace Molybdenum Wire Vacuum Sintering Furnace
- Multi Heating Zones CVD Tube Furnace Machine for Chemical Vapor Deposition Equipment
- Magnesium Extraction and Purification Condensing Tube Furnace
People Also Ask
- What is the specific purpose of pre-treating Terbium chloride hexahydrate? Ensure Purity in Cs3Cu2I5:Tb Synthesis
- What is the maximum temperature capability of the furnace? Find Your Perfect High-Temp Solution
- What role does a microwave chemical reactor play in the synthesis of carbon xerogels? Precision Control & Efficiency
- What is the function of planetary ball mills or industrial mixing granulators prior to RHF? Optimize FMDS Reactivity
- What is the significance of programmed temperature control in TiO2 thin film conversion? Master Structural Precision
- What is the purpose of adding phosphorus pentoxide (P2O5) as a desiccant? Ensure Deep Electrolyte Regeneration
- What is sintering and what types of materials can it be applied to? Unlock Dense, Strong Materials for Your Projects
- How does the pulling and rotation control system of a Czochralski growth furnace affect crystal quality?



















