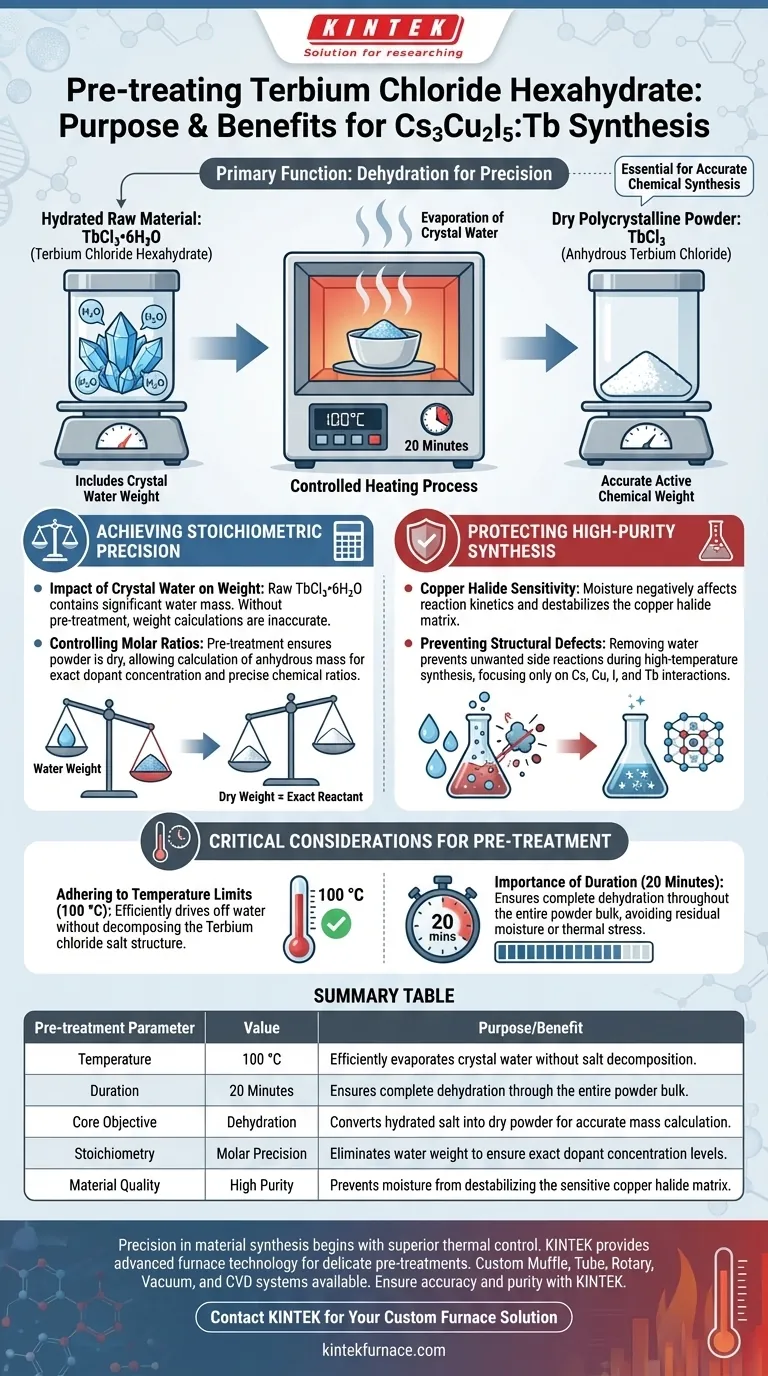
The primary function of pre-treating Terbium chloride hexahydrate (TbCl3•6H2O) in a heating furnace is the complete evaporation of crystal water inherent in the raw material. By subjecting the compound to a temperature of 100 °C for 20 minutes, you transform the hydrated salt into a dry polycrystalline powder essential for accurate chemical synthesis.
Core Insight Dehydration is not merely a cleaning step; it is a calibration step. Removing crystal water prevents moisture from compromising the synthesis of high-purity copper halides and ensures that the mass of the raw material corresponds exactly to the active chemical required for precise molar ratios.

Achieving Stoichiometric Precision
The Impact of Crystal Water on Weight
Raw Terbium chloride often comes in a hexahydrate form, meaning water molecules are bound within the crystal lattice.
If you weigh the raw material without pre-treatment, a significant portion of that mass is water, not the Terbium compound itself. This discrepancy inevitably leads to errors in calculation when determining the amount of material needed for the reaction.
Controlling Molar Ratios
To synthesize Cs3Cu2I5:Tb with specific optical or structural properties, the doping concentration of Terbium must be exact.
Pre-treating the material ensures that the powder is "dry." This allows researchers to calculate and weigh the precursor based on its anhydrous or dehydrated mass, ensuring the final molar ratio of the dopant is chemically accurate.
Protecting High-Purity Synthesis
Copper Halide Sensitivity
The synthesis of copper halides is a delicate process that requires high-purity environments.
Moisture introduced through raw materials can negatively affect the reaction kinetics or the stability of the final product. By eliminating water from the Terbium precursor, you remove a variable that could otherwise destabilize the formation of the copper halide matrix.
Preventing Structural Defects
Retaining moisture during the heating phases of synthesis can lead to unwanted side reactions.
Evaporating the water beforehand ensures that the subsequent high-temperature synthesis focuses solely on the interaction between the Cesium, Copper, Iodine, and Terbium ions, rather than interacting with steam or residual moisture.
Critical Considerations for Pre-treatment
Adhering to Temperature Limits
While the goal is dehydration, precise temperature control is vital.
The protocol specifies 100 °C because this temperature is sufficient to drive off water molecules without decomposing the Terbium chloride salt itself. Exceeding this temperature unnecessarily risks altering the chemical structure of the dopant.
The Importance of Duration
The specified duration of 20 minutes is a calculated window to ensure completeness.
Heating for a shorter period risks leaving residual moisture in the center of the powder bulk. Conversely, accurate timing ensures the material is fully prepped without wasting energy or exposing the material to thermal stress longer than necessary.
Making the Right Choice for Your Protocol
To ensure the reproducibility of your Cs3Cu2I5:Tb polycrystalline materials, apply the pre-treatment step with the following goals in mind:
- If your primary focus is precise doping accuracy: Verify that the heating cycle is completed fully so that your weight measurements reflect the reactant, not the solvent.
- If your primary focus is material purity: Strictly adhere to the pre-treatment phase to prevent moisture-induced degradation of the sensitive copper halide lattice.
Eliminating variables at the raw material stage is the most effective way to guarantee the quality of your final polycrystalline product.
Summary Table:
| Pre-treatment Parameter | Value | Purpose/Benefit |
|---|---|---|
| Temperature | 100 °C | Efficiently evaporates crystal water without salt decomposition. |
| Duration | 20 Minutes | Ensures complete dehydration through the entire powder bulk. |
| Core Objective | Dehydration | Converts hydrated salt into dry powder for accurate mass calculation. |
| Stoichiometry | Molar Precision | Eliminates water weight to ensure exact dopant concentration levels. |
| Material Quality | High Purity | Prevents moisture from destabilizing the sensitive copper halide matrix. |
Precision in material synthesis begins with superior thermal control. KINTEK provides the advanced furnace technology required for delicate pre-treatments like the dehydration of Terbium chloride. Backed by expert R&D and manufacturing, we offer customizable Muffle, Tube, Rotary, Vacuum, and CVD systems tailored for lab high-temp applications. Ensure your stoichiometric accuracy and prevent structural defects with equipment designed for high-purity results. Contact KINTEK today to find your custom furnace solution!
Visual Guide
References
- Haifeng Chen. Study on rare-earth element-doped copper halides. DOI: 10.54254/2977-3903/2025.23781
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
People Also Ask
- How does a vacuum drying oven contribute to stable lithium-selenium battery electrodes? Ensure Purity and Performance
- What is the function of a top-blown oxygen-nitrogen system? Precision Control for High-Yield Smelting
- What are the requirements for sulfur powder loading in MoS2 synthesis? Master the 50-150 mg Precision Range
- How does DTA contribute to determining processing parameters for Ge-Se-Tl-Sb alloys? Optimize Your Thermal Analysis
- Why is a sealed heating vessel used with a stepped heating process to infiltrate sulfur? Maximize Li-S Battery Performance
- What is the function of a Mass Flow Controller (MFC)? Achieve Precise Ethanol Vapor Delivery for Graphene Synthesis
- What is the function of a laboratory high-temperature furnace in eggshell powder pretreatment? Optimize AA6061 Composites
- What is the purpose of using an Argon protective atmosphere during the casting of H13 steel? Boost Purity and Strength



















