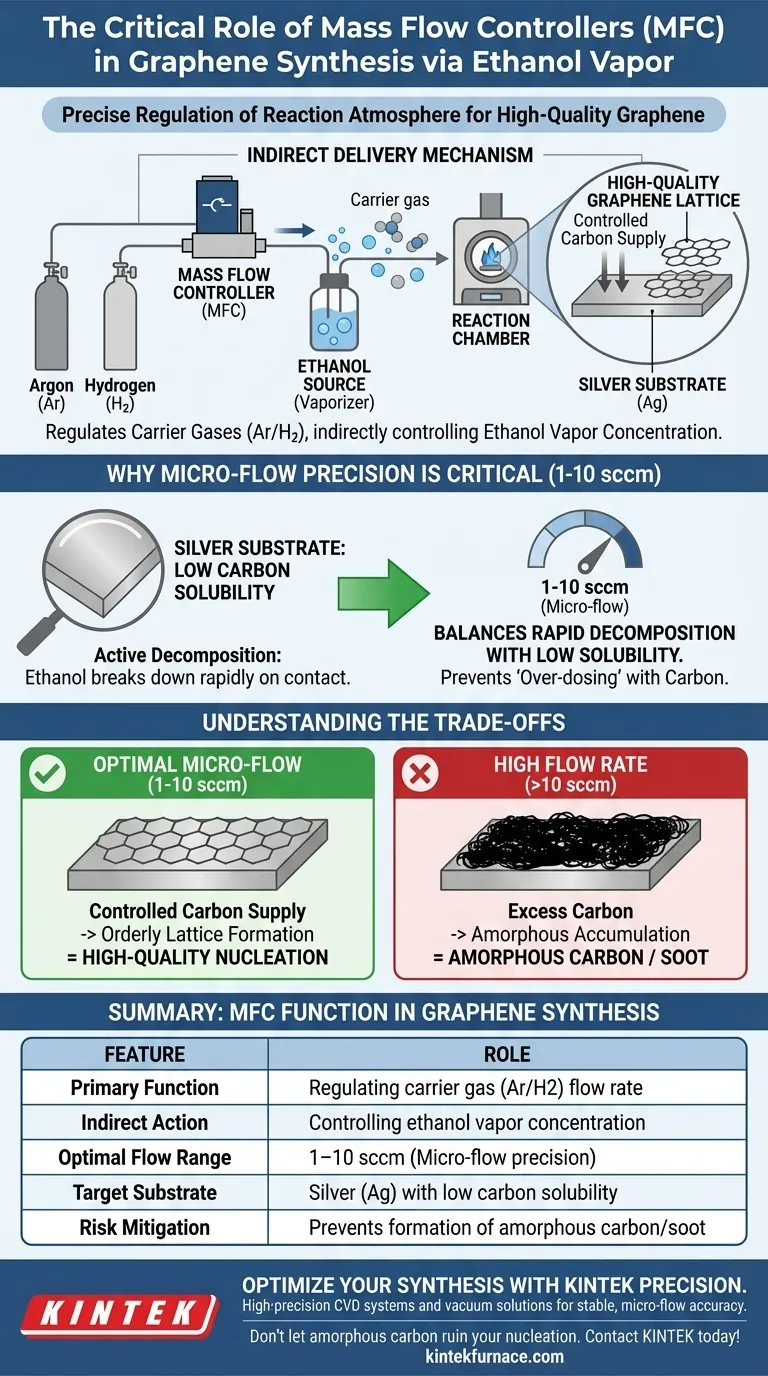
In the context of graphene synthesis, the Mass Flow Controller (MFC) serves as the precise regulator of the reaction atmosphere, specifically managing the delivery of ethanol vapor. It functions by regulating the flow of carrier gases—typically argon or hydrogen—rather than the ethanol itself. By controlling the speed of these carrier gases, the MFC indirectly dictates the exact concentration of ethanol vapor introduced into the reaction chamber.
The Core Takeaway The MFC is the critical barrier against "over-dosing" the reaction chamber with carbon. By maintaining stable micro-flow rates (1–10 sccm), it balances the rapid decomposition of ethanol with the low carbon solubility of the silver substrate, ensuring the growth of high-quality graphene rather than useless amorphous carbon.

The Mechanism of Indirect Delivery
Regulating Carrier Gases
The MFC is not attached to the ethanol source to pump liquid. Instead, it is installed on the gas lines for argon or hydrogen.
These gases act as vehicles. As they flow through the system, they carry ethanol vapor with them.
Controlling Vapor Concentration
The rate at which the carrier gas flows determines how much ethanol vapor reaches the substrate.
Therefore, the MFC's ability to maintain a steady gas flow is the direct lever used to control the ethanol concentration available for the reaction.
Why Micro-Flow Precision is Critical
The Chemistry of Silver Substrates
This process typically uses silver as a catalyst substrate. Silver is unique because it has very low carbon solubility.
Unlike metals that absorb carbon like a sponge, silver holds very little. Consequently, the carbon atoms from the ethanol must settle on the surface immediately.
Managing Active Decomposition
Ethanol decomposes (breaks down) very actively when it comes into contact with the silver surface.
This creates a rapid supply of carbon atoms. If this supply is not strictly limited, the atoms pile up faster than they can organize into a graphene lattice.
The Role of the 1-10 sccm Range
To manage this, the MFC must operate at micro-flow rates, specifically between 1 and 10 sccm (Standard Cubic Centimeters per Minute).
This extremely low flow rate restricts the amount of ethanol entering the chamber, slowing down the carbon supply to a manageable level.
Understanding the Trade-offs
The Risk of High Flow Rates
If the MFC allows the flow rate to exceed the optimal micro-range, the balance is lost.
The active decomposition of ethanol will flood the silver surface with excess carbon.
Formation of Amorphous Carbon
Because the silver cannot absorb this excess and the lattice cannot form fast enough, the carbon accumulates as thick, amorphous carbon.
This results in a disordered, soot-like coating rather than the single-atom-thick, crystalline structure of high-quality graphene.
Making the Right Choice for Your Goal
If your primary focus is High-Quality Nucleation:
- Set your Mass Flow Controller to the lower end of the spectrum (closer to 1 sccm) to strictly limit carbon supply and allow time for orderly lattice formation.
If your primary focus is Process Stability:
- Ensure your MFC is rated specifically for stable operation at low ranges (1-10 sccm), as standard controllers may struggle to maintain precision at these micro-rates.
Precision in carrier gas flow is the only way to prevent rapid ethanol decomposition from ruining the graphene structure.
Summary Table:
| Feature | Role in Graphene Synthesis |
|---|---|
| Primary Function | Regulating carrier gas (Argon/Hydrogen) flow rate |
| Indirect Action | Controlling ethanol vapor concentration |
| Optimal Flow Range | 1–10 sccm (Micro-flow precision) |
| Target Substrate | Silver (Ag) with low carbon solubility |
| Risk Mitigation | Prevents formation of amorphous carbon/soot |
Optimize Your Synthesis with KINTEK Precision
Achieving the perfect graphene lattice requires more than just high temperatures; it demands absolute control over gas dynamics. Backed by expert R&D and world-class manufacturing, KINTEK provides high-precision CVD systems, Tube furnaces, and Vacuum solutions designed to integrate seamlessly with low-flow Mass Flow Controllers.
Whether you are synthesizing graphene on silver or exploring new carbon allotropes, our customizable systems ensure the stability and micro-flow accuracy your research demands. Don't let amorphous carbon ruin your nucleation—Contact KINTEK today to build your custom high-temperature lab solution!
Visual Guide
References
- Hikaru Iwatani, Fumihiko Maeda. Graphene Synthesis on Silver Foil by Chemical Vapor Deposition Using Ethanol. DOI: 10.1380/ejssnt.2025-026
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 304 316 Stainless Steel High Vacuum Ball Stop Valve for Vacuum Systems
- Ultra High Vacuum CF Observation Window Flange with High Borosilicate Glass Sight Glass
- Cylindrical Resonator MPCVD Machine System for Lab Diamond Growth
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- CF KF Flange Vacuum Electrode Feedthrough Lead Sealing Assembly for Vacuum Systems
People Also Ask
- What is the function of a laboratory vacuum drying oven for Fe-N-C catalysts? Preserve Nanoporous Structure
- Why is a precision oven used to dry washed cherry pits? Unlock Superior Activated Carbon Production
- Why is precise control of carrier gas flow rates required for hydrochar activation? Optimize Carbon Yield & Purity
- What necessary conditions does a vacuum drying oven provide for geopolymers? Optimize Your Curing and Molding Process
- What is the purpose of using a vacuum drying oven? Ensure Safety and Accuracy in Mortar Testing
- What is the necessity of baking electrode sheets in a vacuum oven? Ensure Battery Stability and Peak Performance
- What is the benefit of accessing furnace technical guides? Optimize Your Research with Precise Equipment Data
- What are the safety precautions for a heat treatment furnace? A Systematic Guide to Protecting Your Lab



















