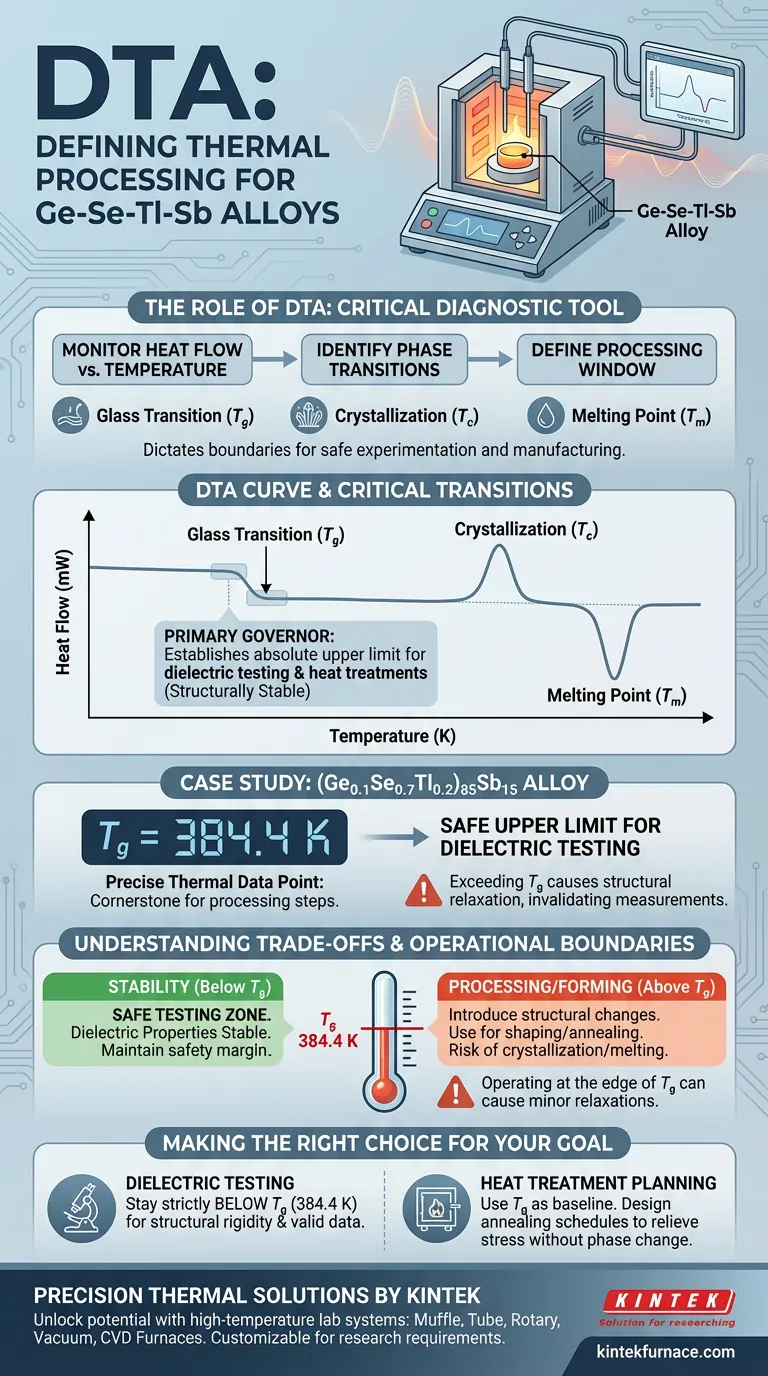
Differential Thermal Analysis (DTA) serves as the critical diagnostic tool for defining the thermal processing window of Ge-Se-Tl-Sb alloys. By precisely monitoring heat flow relative to temperature changes, DTA identifies the material's fundamental phase transitions—specifically the glass transition temperature ($T_g$), crystallization temperature ($T_c$), and melting point ($T_m$)—which dictate the boundaries for safe experimentation and manufacturing.
DTA acts as the primary governor for thermal processing, specifically by identifying the glass transition temperature ($T_g$). This value establishes the absolute upper temperature limit for dielectric testing and heat treatments, ensuring the material remains structurally stable during analysis.

The Role of DTA in Thermal Characterization
Identifying Critical Phase Transitions
To process complex chalcogenide alloys effectively, you must understand exactly where the material changes state.
DTA measures the difference in temperature between the sample and a reference material as they are heated. This reveals endothermic and exothermic events that correspond to specific physical changes.
By mapping these heat flow changes, DTA isolates the Glass Transition Temperature ($T_g$), Crystallization Temperature ($T_c$), and Melting Point ($T_m$).
Establishing Operational Boundaries
The data derived from DTA does more than characterize the material; it sets the safety rules for all subsequent work.
Knowing these transition points prevents you from inadvertently destroying the sample during testing.
Specifically, the onset of the glass transition represents the point where the rigid solid begins to soften, marking the ceiling for most non-destructive tests.
Case Study: The $(Ge_{0.1}Se_{0.7}Tl_{0.2}){85}Sb{15}$ Alloy
Precise Thermal Data Points
For the specific alloy composition $(Ge_{0.1}Se_{0.7}Tl_{0.2}){85}Sb{15}$, DTA provides accurate, quantifiable benchmarks.
Analysis of this alloy identifies a specific $T_g$ of 384.4 K.
This single data point is the cornerstone for determining how the material can be handled in subsequent processing steps.
Implications for Dielectric Testing
The $T_g$ value of 384.4 K serves a specific practical purpose: it defines the safe upper temperature limit for dielectric property testing.
If testing temperatures exceed this limit, the material structure relaxes, invalidating dielectric measurements.
Therefore, DTA provides the critical guidance required to plan heat treatments that modify material properties without inducing unwanted phase changes.
Understanding the Trade-offs
The Risk of Thermal Proximity
While DTA provides a precise limit (e.g., 384.4 K), operating right at the edge of this limit is a common pitfall.
Processing or testing too close to the $T_g$ can introduce minor structural relaxations even if the material has not fully transitioned.
Stability vs. Processing Needs
There is an inherent tension between testing stability and processing requirements.
To shape or mold the glass, you must exceed $T_g$, but to test its stable dielectric properties, you must stay strictly below it. DTA clarifies exactly where that line is drawn, but it is up to the operator to maintain a safety margin.
Making the Right Choice for Your Goal
Using the thermal parameters derived from DTA allows for precise control over the alloy's lifecycle.
- If your primary focus is Dielectric Testing: Ensure all experimental protocols are conducted strictly below the $T_g$ (384.4 K) to maintain structural rigidity and measurement validity.
- If your primary focus is Heat Treatment Planning: Use the $T_g$ as a baseline to design annealing schedules that relieve stress without triggering crystallization or melting.
By adhering to the limits established by DTA, you ensure the physical integrity of the alloy is preserved throughout the testing and manufacturing process.
Summary Table:
| Transition Point | Symbol | Significance for Ge-Se-Tl-Sb | Example Value (K) |
|---|---|---|---|
| Glass Transition | Tg | Defines safe upper limit for dielectric testing | 384.4 |
| Crystallization | Tc | Indicates onset of exothermic phase change | N/A |
| Melting Point | Tm | Represents the boundary for liquid phase transition | N/A |
| Safety Margin | - | Prevents structural relaxation during heat treatment | < Tg |
Precision Thermal Solutions for Advanced Material Science
Unlock the full potential of your Ge-Se-Tl-Sb alloys with industry-leading thermal processing equipment. Backed by expert R&D and world-class manufacturing, KINTEK offers a comprehensive range of high-temperature lab systems including Muffle, Tube, Rotary, Vacuum, and CVD furnaces, all fully customizable to meet your unique research requirements.
Whether you are conducting dielectric testing below the glass transition temperature or designing complex heat treatment cycles, KINTEK provides the reliability and temperature uniformity your lab demands.
Ready to elevate your thermal analysis? Contact KINTEK today to consult with our experts!
Visual Guide
References
- A. M. Ismail, E.G. El-Metwally. Insight on the optoelectronic properties of novel quaternary Ge–Se–Tl–Sb non-crystalline glassy alloy films for optical fiber sensing devices. DOI: 10.1140/epjp/s13360-024-05012-6
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant Rotating Furnace
- Laboratory Quartz Tube Furnace RTP Heating Tubular Furnace
People Also Ask
- What is the role of industrial electric drying ovens in FDSSC titanium photoanode treatment? Enhance Solar Efficiency
- What role does an ultrafast Joule heating device play in the synthesis of heterostructure nanocatalysts?
- How does a stable constant temperature environment influence the structural development of LDHs during aging?
- What is the impact of microwave power on the synthesis of 2D metal oxides? Master High-Speed Material Production
- What is the significance of the 220 °C annealing process? Unlock High-Purity Anti-Perovskite Thin Film Synthesis
- What role does a laboratory vacuum drying oven play in the post-treatment process of porous carbon derived from polyethylene terephthalate (PET)? Crucial for preserving material structure.
- How does the QIO algorithm improve temperature control precision in electric furnaces? Achieve Global Optimization
- What roles does a laboratory constant-temperature drying oven play in evaluating eggshell adsorbents? Key Insights


