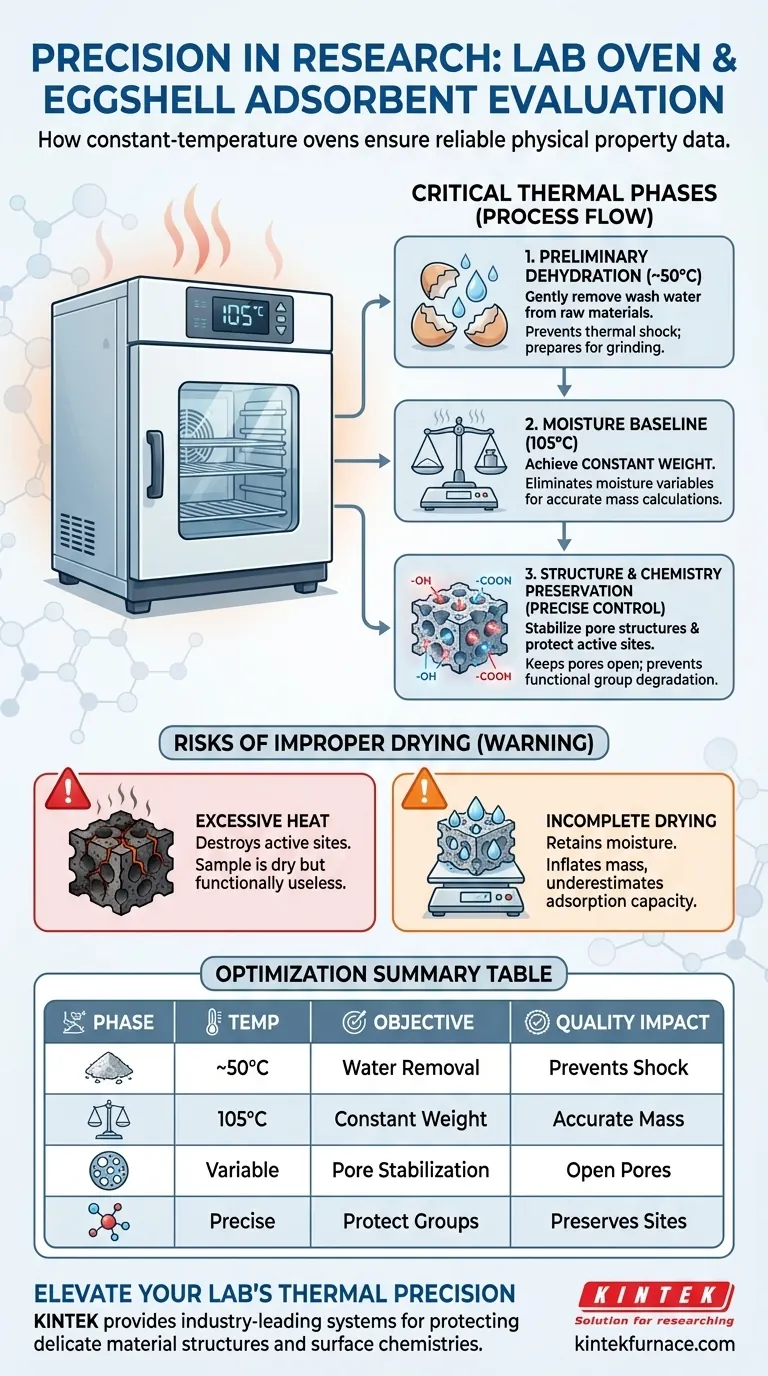
A laboratory constant-temperature drying oven serves as a fundamental instrument for ensuring the reliability of eggshell adsorbent studies. It manages critical thermal processes ranging from the preliminary dehydration of washed raw materials to the precise 105°C environment required for moisture content analysis and final structural stabilization.
The drying oven acts as a control mechanism for material integrity, ensuring that moisture removal does not compromise the adsorbent's pore structure or surface functional groups. Its precise temperature regulation is the baseline for achieving constant weight and reproducible experimental data.
The Critical Phases of Thermal Processing
Preliminary Dehydration of Raw Materials
After the initial washing process to remove organic residues from the eggshells, the material must be dried to a manageable state.
The constant-temperature oven is used to gently dry these raw materials, often at lower temperatures such as 50°C.
This step is essential to prepare the raw stock for grinding or chemical modification without inducing thermal shock or degradation.
Establishing Accurate Moisture Baselines
To evaluate physical properties accurately, you must know the exact dry mass of your sample.
The oven provides a stable environment, typically at 105°C, to remove intrinsic moisture until the sample reaches a constant weight.
This standardizes the sample, allowing for precise calculations of adsorption capacity by eliminating water weight as a variable.
Preserving Adsorbent Structure and Chemistry
Stabilizing Pore Structures
During the preparation of activated adsorbents, the final drying phase is not merely about water removal; it is about structural enforcement.
The oven performs the final drying of activated adsorbents to maintain the stability of the pore structures.
Proper drying ensures these pores remain open and accessible, which is vital for the physical entrapment of contaminants during adsorption.
Protecting Surface Functional Groups
Eggshell adsorbents rely on specific surface chemistry to bind pollutants.
Precise temperature control prevents thermal damage to sensitive surface functional groups, such as hydroxyl and carboxyl groups.
Maintaining these active sites is critical, as overheating could denature the surface and significantly reduce the material's chemical adsorption efficiency.
Understanding the Risks of Improper Drying
Thermal Degradation of Active Sites
While heat is necessary for drying, excessive heat is destructive to organic-based adsorbents like eggshells.
If the oven temperature fluctuates or exceeds the optimal range, you risk destroying the adsorption active sites.
This leads to a paradox where a sample is perfectly dry but functionally useless because the chemical groups required for binding have been neutralized.
The Consequence of Incomplete Drying
Conversely, failing to reach a constant weight creates significant data errors.
If the sample retains moisture due to insufficient drying time or temperature, your weight measurements will be artificially high.
This inflates the apparent mass of the adsorbent, causing you to underestimate its specific adsorption capacity per gram.
Optimizing Your Drying Protocol
To ensure valid physical property evaluation of eggshell adsorbents, align your oven settings with your specific experimental phase:
- If your primary focus is Raw Material Preparation: set the oven to approximately 50°C to gently remove wash water without altering the shell matrix.
- If your primary focus is Quantitative Analysis: utilize a stable 105°C setting to drive off all moisture until the sample weight stabilizes (constant weight).
- If your primary focus is Surface Chemistry Preservation: strictly monitor temperature limits to protect hydroxyl and carboxyl groups from thermal degradation.
Precision in drying is the invisible variable that determines the reproducibility of your adsorption data.
Summary Table:
| Drying Phase | Temperature Setting | Primary Objective | Impact on Adsorbent Quality |
|---|---|---|---|
| Raw Material Dehydration | ~50°C | Preliminary water removal | Prevents thermal shock; prepares for grinding |
| Quantitative Analysis | 105°C | Achieve constant weight | Eliminates moisture variables for accurate mass |
| Structural Stabilization | Variable (Controlled) | Pore structure enforcement | Keeps pores open and accessible for adsorption |
| Chemical Preservation | Precise Regulation | Protect functional groups | Prevents degradation of hydroxyl and carboxyl groups |
Precision Thermal Processing for Your Research
Achieving reproducible data in adsorbent studies requires the highest level of temperature stability. KINTEK provides industry-leading Muffle, Tube, Rotary, Vacuum, and CVD systems, specifically engineered to protect delicate material structures and surface chemistries.
Backed by expert R&D and manufacturing, our lab high-temp furnaces are fully customizable to meet the unique requirements of your eggshell adsorbent research or any advanced material application.
Ready to elevate your lab's thermal precision? Contact KINTEK today for a customized solution and ensure your materials reach their peak performance.
Visual Guide
References
- Hesty Nuur Hanifah, Diyanti Alma Kusuma Dani. Comparison of the Effectiveness of Calcined Chicken and Duck Eggshells as Zn Metal Adsorbent Using Atomic Absorption Spectrophotometric. DOI: 10.22146/ijc.74930
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
People Also Ask
- What processing conditions does an industrial heating furnace provide during hot forging? Optimize Fe-Mn-Si Alloys
- Why is precise heating rate control necessary? Master Activated Carbon Heat Treatment with KINTEK
- How does the control of high-purity Argon gas flow affect Al/Ni film deposition? Master Precision Sputtering
- What role does an industrial-grade walking beam heating furnace play in SSC bars? Ensure Optimal Metallurgical Bonding
- What is the purpose of heating a precursor solution to 80 °C and 300 rpm stirring? Achieve High-Entropy Uniformity
- What role does the high-temperature boiling step play in rice husk silica conversion? Boost Your Extraction Yields
- What are the advantages of PVD coating? Achieve Precision, Purity, and Eco-Friendly Performance
- How do high-temperature annealing furnaces ensure equilibrium in Bi2Se3-Nd2Se3 alloys? Expert Thermal Control Solutions



















