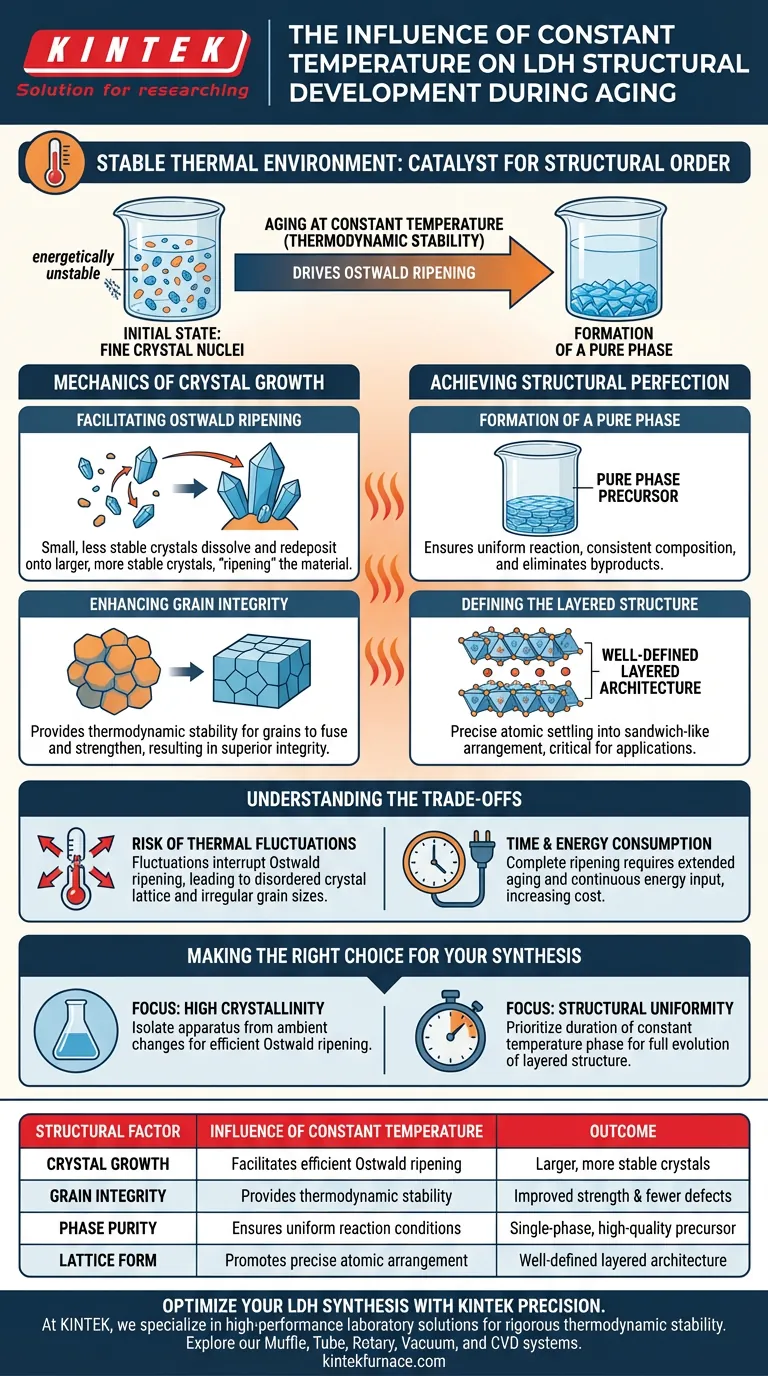
A stable thermal environment is the catalyst for structural order. When aging Layered Double Hydroxides (LDHs), maintaining a constant temperature is essential for converting the initial fine crystal nuclei into robust structures. This specific environmental condition drives Ostwald ripening, which directly improves grain integrity and ensures the material develops into a pure phase precursor with a well-defined layered architecture.
Thermodynamic stability during the aging process is the primary mechanism for achieving high-quality LDH crystals. By maintaining a constant temperature, you facilitate the controlled growth of grains, preventing structural defects and ensuring the formation of a distinct, uniform layered lattice.
The Mechanics of Crystal Growth
Facilitating Ostwald Ripening
The co-precipitation process generates a multitude of fine crystal nuclei. These initial particles are small and energetically unstable.
A constant temperature environment allows Ostwald ripening to occur efficiently. In this process, smaller, less stable crystals dissolve and redeposit onto larger, more stable crystals, effectively "ripening" the material into a more mature state.
Enhancing Grain Integrity
Without thermal stability, the growth of these crystals can be chaotic and uneven.
By strictly controlling the temperature, you provide the thermodynamic stability required for the grains to fuse and strengthen. This results in superior grain integrity, making the final material more robust and structurally sound.
Achieving Structural Perfection
Formation of a Pure Phase
Inconsistent temperatures can lead to mixed phases or incomplete crystallization.
A stable environment ensures the reaction proceeds uniformly, leading to a pure phase precursor. This means the chemical composition and crystal structure are consistent throughout the entire sample, eliminating unwanted byproducts.
Defining the Layered Structure
LDHs are defined by their specific "sandwich-like" layered arrangement.
The aging process under constant heat allows the atoms to settle into this typical layered structure with high precision. This structural definition is critical for the material's performance in applications such as catalysis or anion exchange.
Understanding the Trade-offs
The Risk of Thermal Fluctuations
While constant temperature is beneficial, achieving it requires rigorous control.
Any significant fluctuation in temperature can interrupt the Ostwald ripening process. This interruption may result in a distribution of irregular grain sizes or a disordered crystal lattice, compromising the material's final properties.
Time and Energy Consumption
Facilitating complete Ostwald ripening is not an instantaneous process.
Maintaining a constant temperature often requires extended aging periods and continuous energy input. While this yields a superior structure, it increases the time and cost of the synthesis compared to rapid, uncontrolled aging methods.
Making the Right Choice for Your Synthesis
To apply these principles effectively to your LDH project, consider your specific structural requirements:
- If your primary focus is high crystallinity: Ensure your apparatus is isolated from ambient temperature changes to maximize the efficiency of Ostwald ripening.
- If your primary focus is structural uniformity: Prioritize the duration of the constant temperature phase to allow the fine nuclei to fully evolve into a well-defined layered structure.
Mastering the thermal environment during aging is the difference between a disordered mixture and a high-performance engineered material.
Summary Table:
| Structural Factor | Influence of Constant Temperature | Outcome |
|---|---|---|
| Crystal Growth | Facilitates efficient Ostwald ripening | Larger, more stable crystals |
| Grain Integrity | Provides thermodynamic stability | Improved strength & fewer defects |
| Phase Purity | Ensures uniform reaction conditions | Single-phase, high-quality precursor |
| Lattice Form | Promotes precise atomic arrangement | Well-defined layered architecture |
Optimize Your LDH Synthesis with KINTEK Precision
Precision in thermal control is the key to mastering the structural evolution of Layered Double Hydroxides. At KINTEK, we specialize in providing high-performance laboratory solutions backed by expert R&D and manufacturing. Our diverse range of Muffle, Tube, Rotary, Vacuum, and CVD systems is designed to maintain the rigorous thermodynamic stability required for perfect crystal growth.
Whether you need uniform aging environments or customizable high-temperature furnaces, KINTEK offers the reliability your research demands. Contact us today to find the perfect system for your unique synthesis needs.
Visual Guide
References
- Daisy W. Leung, Dermot O’Hare. Optimising the acid–base ratio of Mg–Al layered double oxides to enhance CO<sub>2</sub> capture performance: the critical role of calcination conditions. DOI: 10.1039/d4dt00270a
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 2200 ℃ Graphite Vacuum Heat Treat Furnace
- 915MHz MPCVD Diamond Machine Microwave Plasma Chemical Vapor Deposition System Reactor
- 9MPa Air Pressure Vacuum Heat Treat and Sintering Furnace
- Vacuum Heat Treat Sintering Furnace Molybdenum Wire Vacuum Sintering Furnace
- Spark Plasma Sintering SPS Furnace
People Also Ask
- Why are carbon nanotubes considered superior adsorbents? Efficient Removal of Harmful Organic Gases
- What functions does ammonia (NH3) perform beyond acting as a nitrogen source? Unlock Advanced Surface Engineering
- Why is the adsorption of dioxins more effective using carbon nanotubes (CNTs)? 3x Superior Efficiency Explained
- Why is a laboratory vacuum evaporation system essential for the preparation of electrodes in high-performance solar cells?
- Why is a high-temperature sintering furnace critical for BCZT ceramics? Achieving High Densification and Performance
- How does the 1600°C range influence biomass microstructure? Transform Carbon into High-Performance Graphite
- What is the purpose of performing a quenching treatment? Optimize Doped Alkali Halide Crystal Spectral Analysis
- Why is a laboratory vacuum oven required for GO slurry? Preserving Chemical Integrity in Graphene Oxide Dehydration



















