High-power microwave irradiation fundamentally alters the kinetics of solid-state synthesis. When applied to 2D transition metal oxides, specifically at industrial levels (e.g., 1000 W), it delivers intense thermal energy instantaneously. This rapid energy injection breaks down precursor bonds and accelerates oxidation, drastically shortening reaction times compared to conventional heating.
The application of high-power microwave energy shifts the synthesis process from a slow thermal ramp to an instantaneous reaction. By delivering localized, high-intensity heat, it rapidly severs precursor bonds and forces immediate oxidation, enabling the quick formation of 2D transition metal oxides.
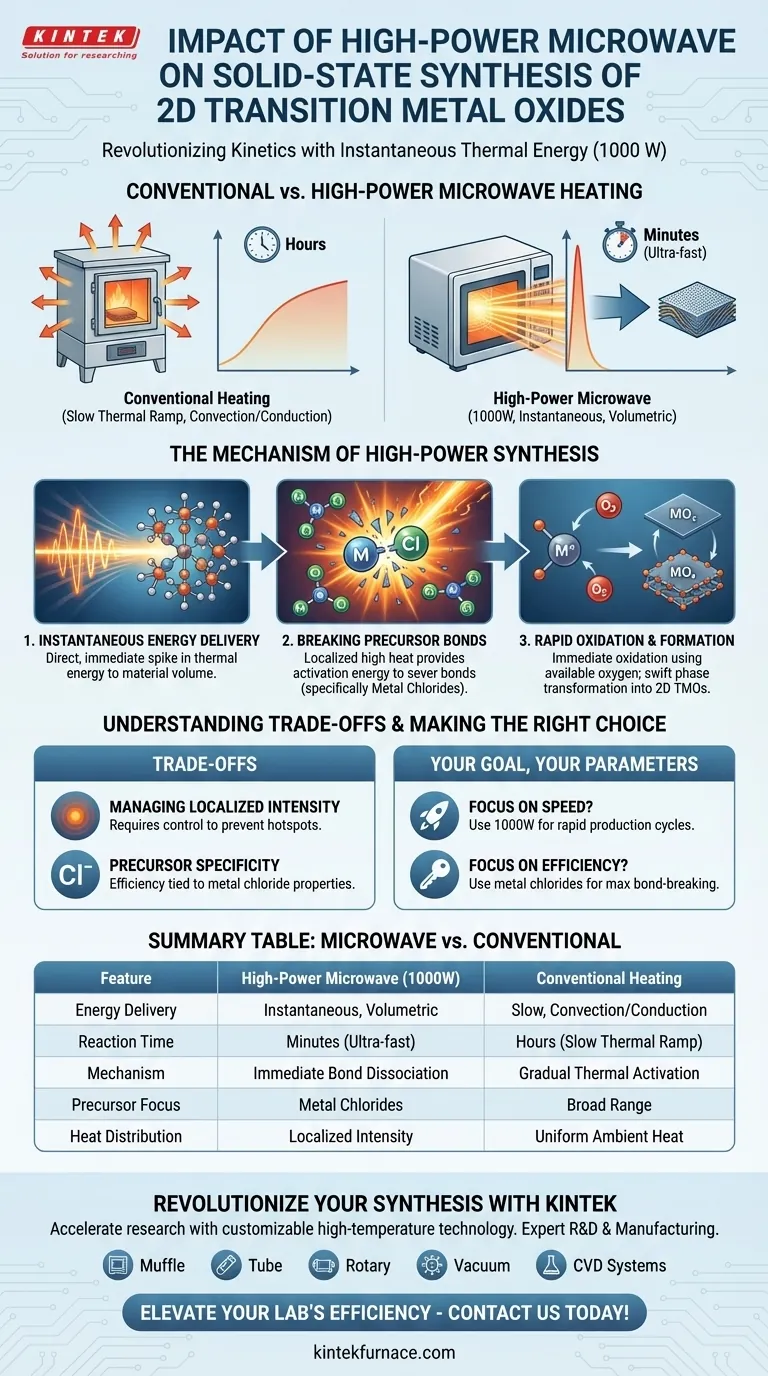
The Mechanism of High-Power Synthesis
Instantaneous Energy Delivery
The core impact of using high-power systems, such as 1000 W industrial-grade units, is the generation of intense thermal energy.
Unlike conventional furnaces that rely on convection or conduction, microwaves deliver energy directly to the material volume.
This results in an immediate spike in thermal energy rather than a gradual increase.
Breaking Precursor Bonds
The specific target of this energy is the chemical structure of the starting material.
The process typically utilizes metal chloride precursors.
The localized high heat provides the activation energy required to rapidly sever the chemical bonds within these metal chlorides.
Driving the Chemical Transformation
The Oxidation Process
Once the precursor bonds are broken, the metal atoms are highly reactive.
The high thermal energy induces immediate oxidation reactions.
These reactions utilize available oxygen molecules found in the surrounding air or within residual moisture in the system.
Rapid Material Formation
The combination of bond breaking and oxidation results in a swift phase transformation.
The precursors are converted into the desired metal oxides in a fraction of the time required by traditional methods.
This speed is the defining characteristic of high-power microwave solid-state synthesis.
Understanding the Trade-offs
Managing Localized Intensity
The primary reference notes that the thermal energy is "localized."
While this allows for rapid heating, it requires careful management to ensure the reaction propagates through the entire sample volume.
Without proper control, "localized" heating can lead to hotspots rather than a uniform transformation.
Precursor Specificity
The process is described specifically in the context of metal chloride precursors.
This implies that the efficiency of bond breaking at these power levels is tied to the specific chemical properties of chlorides.
Using different precursors may not yield the same rapid bond dissociation or oxidation efficiency.
Making the Right Choice for Your Goal
To effectively utilize high-power microwave synthesis, align your parameters with your specific material objectives.
- If your primary focus is Speed: Utilize high-power settings (1000 W) to leverage instantaneous thermal energy for rapid production cycles.
- If your primary focus is Reaction Efficiency: Ensure you are using metal chloride precursors to maximize the bond-breaking potential of the microwave energy.
High-power microwave synthesis offers a pathway to rapid material production by replacing slow thermal ramping with immediate, bond-breaking thermal intensity.
Summary Table:
| Feature | High-Power Microwave (1000W) | Conventional Heating |
|---|---|---|
| Energy Delivery | Instantaneous, Volumetric | Slow, Convection/Conduction |
| Reaction Time | Minutes (Ultra-fast) | Hours (Slow Thermal Ramp) |
| Mechanism | Immediate Bond Dissociation | Gradual Thermal Activation |
| Precursor Focus | Metal Chlorides | Broad Range |
| Heat Distribution | Localized Intensity | Uniform Ambient Heat |
Revolutionize Your Material Synthesis with KINTEK
Accelerate your research and production cycles with cutting-edge high-temperature technology. Backed by expert R&D and manufacturing, KINTEK offers high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems, all customizable to meet your unique laboratory requirements. Whether you are optimizing 2D transition metal oxide synthesis or scaling industrial processes, our advanced furnace solutions provide the precision and power you need.
Ready to elevate your lab's efficiency? Contact us today to discuss your custom furnace needs!
Visual Guide
References
- Muxuan Yang, Weinan Xu. Scalable solid-state synthesis of 2D transition metal oxide/graphene hybrid materials and their utilization for microsupercapacitors. DOI: 10.1039/d4nr00587b
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Inclined Rotary Plasma Enhanced Chemical Deposition PECVD Tube Furnace Machine
- 915MHz MPCVD Diamond Machine Microwave Plasma Chemical Vapor Deposition System Reactor
- Multi Heating Zones CVD Tube Furnace Machine for Chemical Vapor Deposition Equipment
- 1400℃ Muffle Oven Furnace for Laboratory
- Multi Zone Laboratory Quartz Tube Furnace Tubular Furnace
People Also Ask
- Why is it important to choose the right type of heat treatment furnace? Boost Efficiency and Quality in Your Lab or Facility
- What is the purpose of using a vacuum drying oven? Maximize Drug Loading & Preserve Mesoporous Silica Nanoparticles
- How are laboratory ovens and analytical balances used for banana powder moisture content? Precision Testing Guide
- Why is a constant temperature drying oven necessary for CN/BOC-X composites? Ensure High Photocatalytic Activity
- How does sodium metal function as a flux? Enhancing Sr-Ge-N Synthesis with Liquid-Phase Dynamics
- What is the purpose of the constant-temperature circulation phase? Ensure Moso Bamboo Integrity with KINTEK Solutions
- What is the basic principle of a sintering furnace? Transform Powder into Dense, Strong Components
- What are the two key phenomena essential to understanding induction heating? Master the Core Principles
















