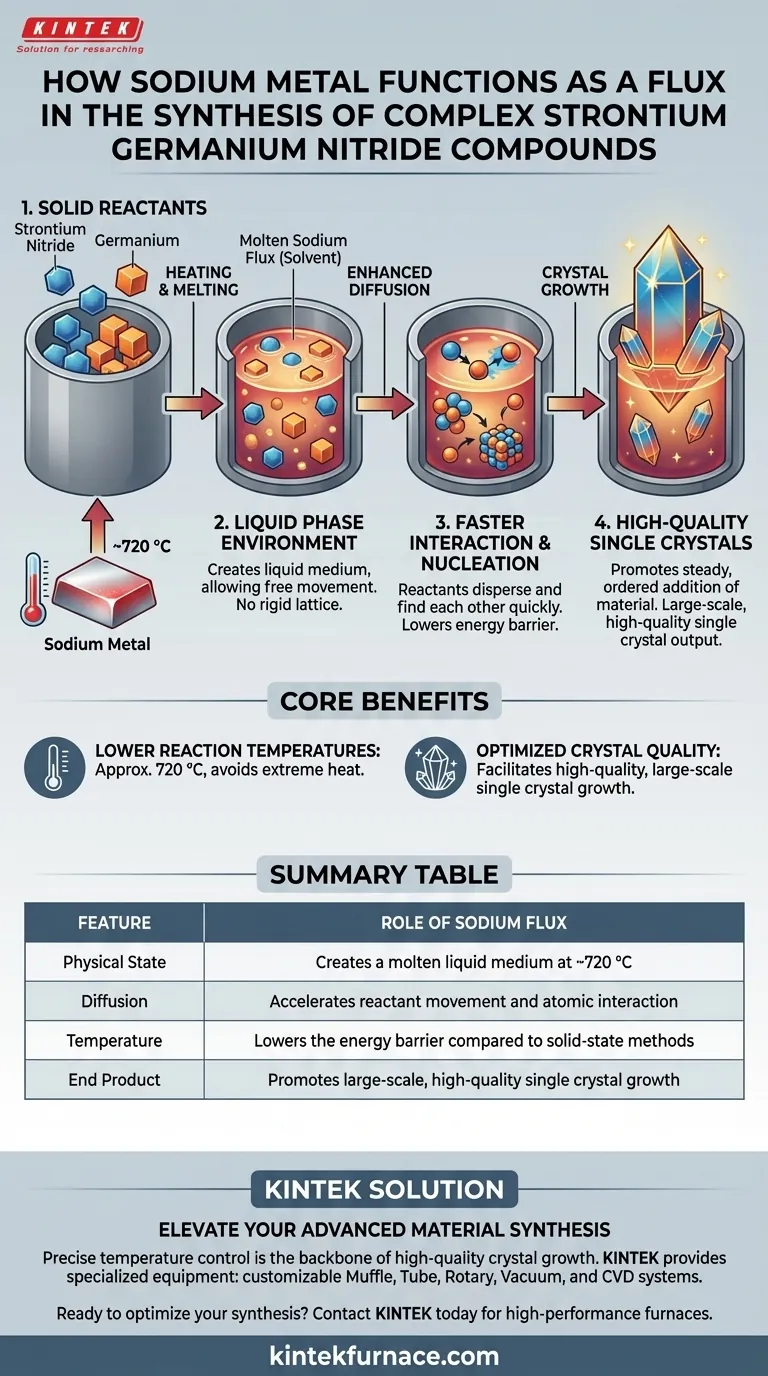
Sodium metal functions as a facilitator for liquid-phase synthesis. It acts as a solvent that melts to create a liquid environment, allowing solid reactants like strontium nitride and germanium to dissolve and interact more freely. This process significantly lowers the energy barrier required for the reaction, enabling the formation of complex compounds at approximately 720 °C.
Core Takeaway: By introducing a liquid phase, sodium flux enhances reactant diffusion and lowers the processing temperature, directly enabling the nucleation and growth of high-quality, large-scale single crystals.

The Mechanics of Sodium Flux
Creating a Liquid Phase Environment
In solid-state chemistry, reacting two solids is often difficult because atoms move slowly. Sodium metal solves this by acting as a liquid-phase flux.
When heated, the sodium melts and surrounds the solid reactants. This creates a medium where the components are no longer locked in a rigid lattice but are free to move.
Enhancing Reactivity and Diffusion
The primary benefit of this liquid environment is the drastic improvement in diffusion rates.
Reactants such as strontium nitride and germanium can disperse through the molten sodium. This increased mobility allows the distinct elements to find one another and react much faster than they would in a traditional solid-state mixture.
Crystal Growth and Thermal Benefits
Lowering Reaction Temperatures
Traditional synthesis often requires extreme heat. The sodium flux method allows synthesis to occur at a relatively low reaction temperature of approximately 720 °C.
Because the flux facilitates mixing at the atomic level, the system does not require excessive thermal energy to force the reactants together.
Facilitating Nucleation
The sodium flux provides an ideal environment for nucleation, the initial step in crystal formation.
Once the reaction begins, the flux supports the steady, ordered addition of material to the growing crystal structure. This results in the production of high-quality, large-scale single crystals rather than disordered powders.
Understanding Process Requirements
Specific Temperature Control
While the temperature is "low" relative to other methods, the process relies heavily on maintaining the specific environment around 720 °C.
Success depends on achieving and sustaining this temperature to ensure the flux remains effective and the diffusion rates are optimized for the specific reactants involved.
Making the Right Choice for Your Synthesis
If you are evaluating synthesis methods for strontium germanium nitride compounds, consider your specific end-goal requirements.
- If your primary focus is Crystal Quality: The sodium flux method is ideal as it facilitates the growth of high-quality, large-scale single crystals through controlled nucleation.
- If your primary focus is Thermal Budget: This method is superior because it enables reactivity at a relatively low temperature (approx. 720 °C), avoiding the need for extreme heat.
The sodium flux method effectively bridges the gap between solid reactants and high-quality crystal output by leveraging liquid-phase dynamics.
Summary Table:
| Feature | Role of Sodium Flux |
|---|---|
| Physical State | Creates a molten liquid medium at ~720 °C |
| Diffusion | Accelerates reactant movement and atomic interaction |
| Temperature | Lowers the energy barrier compared to solid-state methods |
| End Product | Promotes large-scale, high-quality single crystal growth |
Elevate Your Advanced Material Synthesis with KINTEK
Precise temperature control is the backbone of high-quality crystal growth. Whether you are utilizing sodium flux methods or traditional solid-state reactions, KINTEK provides the specialized equipment needed to succeed. Backed by expert R&D and manufacturing, we offer customizable Muffle, Tube, Rotary, Vacuum, and CVD systems designed to meet the rigorous demands of lab high-temp research.
Ready to optimize your synthesis process? Contact KINTEK today to discover how our high-performance furnaces can bring precision and efficiency to your unique research needs.
Visual Guide
References
- Lukas Link, Rainer Niewa. Nitridogermanates(IV): The Germanide Oxide Sr<sub>15</sub>Ge[GeN<sub>4</sub>]<sub>3</sub>O, the Carbodiimide Ba<sub>5</sub>[GeN<sub>4</sub>][CN<sub>2</sub>], and the Oxidonitridogermanate Sr<sub>6</sub>[Ge<sub>2</sub>N<sub>6</sub>O]. DOI: 10.1002/zaac.202500068
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- Vacuum Heat Treat Sintering Furnace Molybdenum Wire Vacuum Sintering Furnace
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- Molybdenum Disilicide MoSi2 Thermal Heating Elements for Electric Furnace
People Also Ask
- What is the significance of the vacuum oven drying process in the preparation of MnO@WAC electrode sheets? Expert Guide
- What is the disadvantage of dental ceramic? Weighing Cost, Strength, and Aesthetics
- How does temperature control affect nanoporous copper dealloying? Master Pore Uniformity and Size
- What is the primary function of compacting PVC and metal oxide mixtures? Enhancing Dechlorination Efficiency
- What role does active carbon play in CaS:Eu2+ phosphor synthesis? Key to Activating High-Efficiency Luminescence
- Why is an industrial electric drying oven required for catalyst support precursors? Secure Pore Integrity
- Why is a vacuum drying oven necessary for Fe-CN@CoCN precursors? Preserve MOF Structural Integrity
- What are the functions of a rotary evaporator and a vacuum drying oven in LTO sol-gel? Optimize Your Synthesis Process












